On August 6th, 2012 the automatic Mars Science Laboratory rover named Curiosity landed on Mars. One of the scientific instruments on board is ChemCam, which has a pulsed laser capable of ablating a focused spot on a remote sample to create a glowing plasma plume of target material. Light from plasma is collected by rover’s telescope on a mast, and the optical spectra are then analyzed by an internal spectrometer. ChemCam can take thousands of spectra per day from a distance of about 7 meters, thus making chemical analyses on the surface of Mars with unprecedented speed. Operationally, the ChemCam data facilitates decisions of where the rover should be driven.

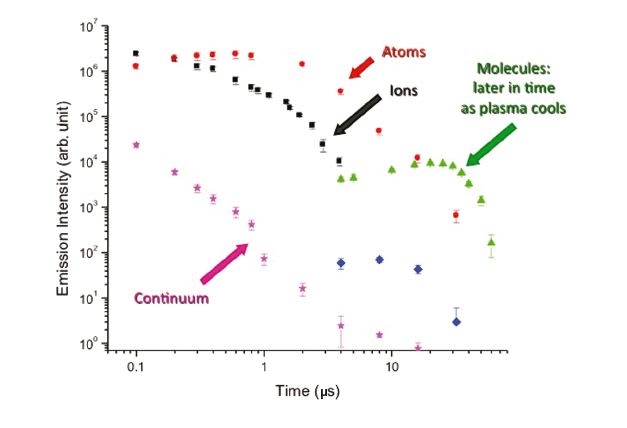
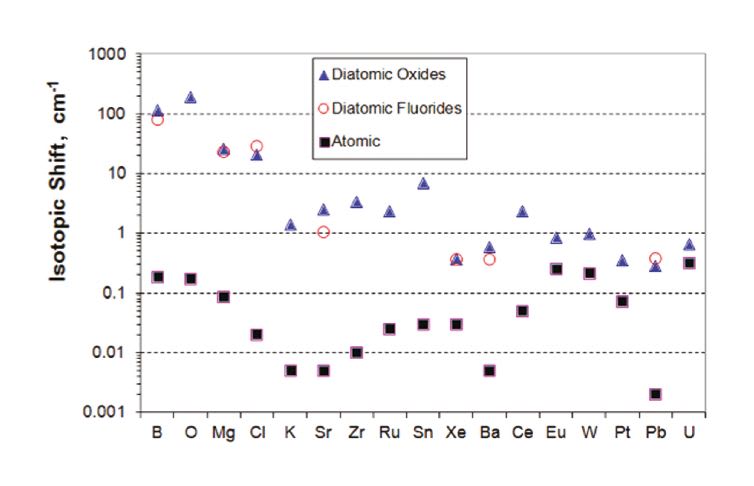
In 2009, Applied Spectra was awarded a NASA SBIR grant to develop an innovative technology that enables not only chemical elemental but also isotopic analysis using ChemCam or a similar instrument. Applied Spectra collaborated in this work with the Lawrence Berkeley National Laboratory. As a result of the joint research efforts, a new elegant technology was born and branded “Laser Ablation Molecular Isotopic Spectrometry” or LAMIS.[1-4] LAMIS shares all the same technical benefits of its predecessor, Laser Induced Breakdown Spectroscopy (LIBS), including rapid analysis and the elimination of sample preparation. LIBS measures atomic emission spectra during the first microsecond after an ablation pulse. LAMIS measurement follows later when the plasma cools down. Then molecules form in the plasma and the intensity of molecular spectra increases and persists for some time (Figure 1). Mo l e cul a r spectra are useful for isotopic analysis because the isotopic shifts in molecular emission are significantly larger than in atomic spectra (Figure 2). The difference in isotopic masses has only a small effect on electronic transitions (as in atoms) but appreciably affects the vibrational and rotational energy levels in molecules.[4] There is no need for a large, high-resolution spectrometer; a compact spectrometer can resolve isotopic spectra. LIBS and LAMIS techniques can be accomplished on the same instrument, thus extending ChemCam with a new dimension of isotopic analysis.
The merits of LAMIS were quickly recognized within the scientific community. LAMIS achieves rapid and direct chemical and isotopic characterization without any acidic dissolution or deep evacuation of samples as required in typical mass spectrometry. Furthermore, rasterizing surface scans and depth profiling are easily realized with high spatial definition (~10nm in depth and ~10μm lateral).
Isotopic analyses have proven to be a powerful scientific tool. Isotopes formed at the origin of the Universe and are produced in stars including catastrophic events of supernovae. Studies of the sources and driving mechanisms of isotopic variations can provide answers to fundamental questions on the development and evolution of stars and planets, as well as our solar system. Large fractionation in stable isotopes of H, C, N, O can be particularly indicative of a range of diverse processes in the biosphere, hydrosphere and lithosphere. Life processes lead to distinctive isotope patterns thereby providing clues to the origin of life and evolution in a galactic context. Isotopic information in paleoclimatology plays a critical role for the reconstruction of variations in past climate conditions. Consequently, isotopic records hold keys to the prediction of future climate changes that may influence global temperature, energy needs, availability of drinking water, and food supplies.
“Overarching issues that could have a significant impact on... strategies for Mars include the absolute chronology of the planet.” This assertion was strongly emphasized in the National Research Council review Assessment of NASA’s Mars Architecture 2007—2016. Radioisotopic age dating is the primary method in which accurate geochronological ages can be established. Measurements of the isotopic ratios of 87Rb/86Sr and 87Sr/86Sr isotopes using LAMIS can provide the age at which rocks solidified by applying the well-known radiometric isochron dating method. Presently, the dissolution and chromatographic separation of strontium from rubidium is necessary prior to conventional mass spectrometric analysis because of nonresolvable isobaric interference between the 87Sr and 87Rb. The results of LAMIS measurements of 86Sr, 87Sr and 88Sr isotopes were recently published,[1,2] while optical spectra of 85Rb and 87Rb in laser ablation plasmas were obtained earlier.[5] Accordingly, a ChemCam-like de vice can potentially be used for age determination. Until now, there was no means by which to make direct age dating measurements on other planets (indirect age estimates for Mars have uncertainties in billions of years, the validity of which is unknown).
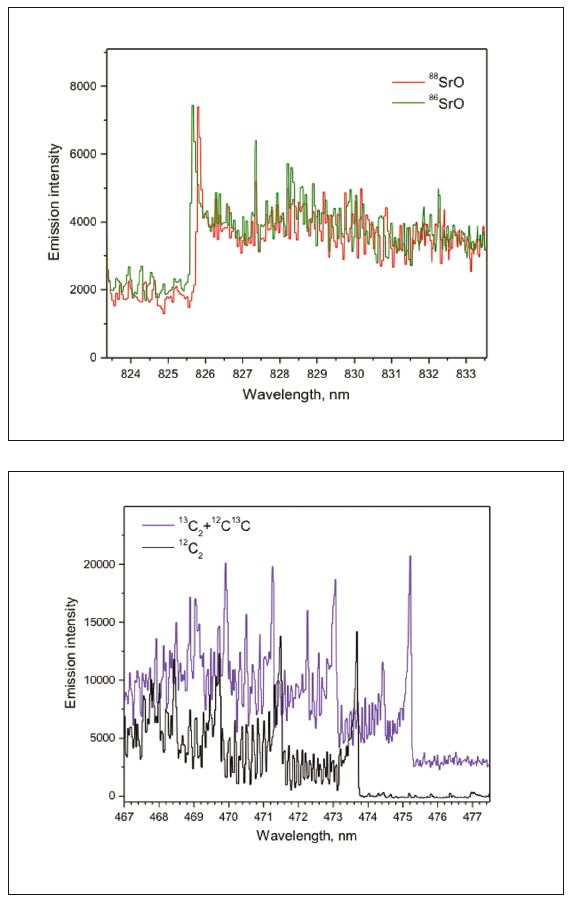
Examples of laser ablation spectra of diatomic radicals SrO generated from isotope-enriched SrCO3 pellets and C2 molecules from samples of graphite and 13C powder are presented in Figure 3. These spectra were collected using a compact echelle spectrograph EMU-65 (Catalina Scientific, Tucson, AZ) fitted with an Electron Multiplying Charge- Coupled Device (EMCCD). Such an instrument would be suitable for space operations. The results demonstrate that isotopes are easily resolvable (shifts of 0.15nm in SrO and 0.7nm in C2). Even partially resolved spectra can be sufficient for the quantitative isotopic detection especially if a range of multiple spectral features, such as rotational lines, is measured at once.[3] Requirements for spectral resolution can be further relaxed when the isotopic ratio is determined using chemometric analysis of spectra. The ability to measure isotope abundance with a low-resolution spectrometer is a significant attribute of LAMIS.
In terrestrial applications LAMIS is poised to speed up, to simplify, and to make isotopic analysis more affordable than at present, although it will remain generally less sensitive than traditional mass spectrometry. Multiple applications of LAMIS are anticipated in the nuclear power industry, medical diagnostics and therapies, forensics, carbon sequestration, ecological and agronomical studies. Several examples of the possible uses are described below to illustrate the breadth of LAMIS applicability.
Owing to its unique nuclear properties, the isotope-enriched 10B is often used in medical technology, particularly in boron neutron capture therapy (BNCT). The neutron capture cross section of 10B is six orders of magnitude higher than that of 11B and is significantly higher than that of any other material. In BNCT, neutron capture by 10B-loaded targeted drugs generates lethal radiation that damages DNA within individual malignant cells. To raise an effective dose, the tumor cells are loaded with 10B at 30—170 μg/g in tissue. Three-dimensional isotopic mapping with fine spatial resolution is necessary in boron radiochemotherapeutic cancer research. Presently, isotopic mapping at the scale of tens of microns can be obtained with laser ablation—inductively coupled plasma— mass spectrometry (LA-ICP-MS) or secondary ion mass spectrometry (SIMS), both involving large and expensive instrumentation that requires a vacuum. In contrast, LAMIS can be implemented as a fieldable device. The nominal sample quantity in LAMIS can be less than 1μg (<103 human cells, i.e. less than a cube of 10×10×10 cells), which defines the micro-sampling spatial resolving ability of LAMIS.
Natural and isotope-enriched boron is often utilized as neutron shielding and neutron absorbing materials in nuclear reactors and spent fuel storage pools. Whether borated metals are used as neutron capturers or whether boric acid is added to cooling water, the ability to measure the 10B and 11B content by LAMIS will be particularly useful for monitoring. Such monitoring can be achieved remotely, delivering a laser beam through a small window and collecting optical emission either by a telescope or a cable of optical fiber.
The application of LAMIS for localized boron isotope determination would enable the development of handheld semiconductor-based neutron sensors in which a layer of 10B-enriched boron carbide with thickness of 1.5 to 100μm is deposited on top of a regular silicon-based diode. Other neutron detection devices lined, coated, or loaded with 10B can be rapidly tested by LAMIS for isotopic and elemental composition, hetero- or homogeneity, and possible defects for the purpose of quality assessment and quality control. The same is true for the production of 10Benriched borated steel, aluminum and borobond ceramics.
Other considerations: Carbon isotopes are indicative of primary bio-productivity and energy cycling and are, therefore, important for the understanding of biochemistry. The biological enhancement of 12C over 13C can be up to 5%, and is measurable by LAMIS. The stable isotope 15N is often used as a marker, particularly to track the efficiency of fertilizers in agronomy. Measurement of the D/H isotopic ratio is essential in paleoclimatology, material sciences, biological and medical research — among many other areas. Deuterated scintillators are used in neutron detectors, and their analysis can be another area of LAMIS applications. Measurement of oxygen and chlorine isotopes is also feasible — which isotopes are very important in geochemical studies.
Applied Spectra has developed a prototype LIBS instrument for standoff measurements at a distance of 30 meters, and also has participated in 50-meter standoff measurements using LIBS in the field.[6] NASA’s ChemCam can measure LIBS spectra from 7 meters away. Similar standoff distances should be possible for isotopic analysis using LAMIS.
This article was written by Alexander Bolíshakov, Senior Scientist at Applied Spectra, Inc. (Fremont, CA). For more information, please contact Dr. Bo Tshakov at
References
- A.A. Bol’shakov, X. Mao, C.P. McKay, R.E. Russo, “Laser ablation — optical cavity isotopic spectrometer for Mars rovers “ — Proc. SPIE, v. 8385, paper 83850C (2012).
- X. Mao, A.A. Bol’shakov, I. Choi, C.P. McKay, D.L. Perry, O. Sorkhabi, R.E. Russo, “Laser Ablation Molecular Isotopic Spectrometry: Strontium and its isotopes” — Spectrochim. Acta Part B, 66, 767-775
- X. Mao, A.A. Bol’shakov, D.L. Perry, O. Sorkhabi, R.E. Russo, “Laser Ablation Molecular Isotopic Spectrometry: Parameter influence on boron isotope measurements” — Spectrochim. Acta Part B, 66, 604-609 (2011).
- R.E. Russo, A.A. Bol’shakov, X. Mao, C.P. McKay, D.L. Perry, O. Sorkhabi, “Laser Ablation Molecular Isotopic Spectrometry” — Spectrochim. Acta Part B, 66, 99-104
- L.A. King, I.B. Gornushkin, D. Pappas, B.W. Smith, J.D. Winefordner, “Rubidium isotope measurements in solid samples by laser ablation-laser atomic absorption spectroscopy” — Spectrochim. Acta Part B, 54, 1771—1781 (1999).
- A.A. Bol’shakov, J.H. Yoo, C. Liu, J.R. Plumer, R.E. Russo, “Laser-Induced Breakdown Spectroscopy in industrial and security applications” — Appl. Opt., 49, C132-C142 (2010).

