An electrolyte additive has shown promise as a means of increasing the sustainable rates of discharge and, hence, the discharge capacities, of lithium- poly (carbon monofluoride) electrochemical power cells. Lithium- poly (carbon monofluoride) [Li-(CF)n] cells and batteries offer very high specific energies — practical values of about 600 W.h/g and a theoretical maximum value of 2,180 W·h/kg. However, because Li-(CF)n cells and batteries cannot withstand discharge at high rates, they have been relegated to niche applications that involve very low discharge currents over times of the order of hundreds to thousands of hours. Increasing the discharge capacities of Li- (CF)n batteries while maintaining high practical levels of specific energy would open new applications for these batteries.
The present electrolyte additive is a member of a class of fluorinated boronbased compounds that function as anion receptors, helping to increase the discharge capacity in two ways:
- They render LiF somewhat soluble in the non-aqueous electrolyte solution, thereby delaying precipitation until a high concentration of LiF in solution has been reached.
- When precipitation occurs, they promote the formation of large LiF grains that do not conformally coat the cathode.
The net effect is to reduce the blockage caused by precipitation of LiF, thereby maintaining a greater degree of access of electrolyte to the cathode.
The fluorinated boron-based anion receptor most commonly used heretofore — tris(pentafluorophenyl) borane — has been found to sharply increase the viscosities of electrolyte solutions. Increases in viscosities generally contribute to reductions in discharge capacities. In contrast, the present additive — tris(hexafluoroisopropyl) borate — can be mixed with such conventional lithium- cell solvents as propylene carbonate and dimethoxyethane to obtain solutions that have much lower viscosities and accept greater concentrations of LiF.
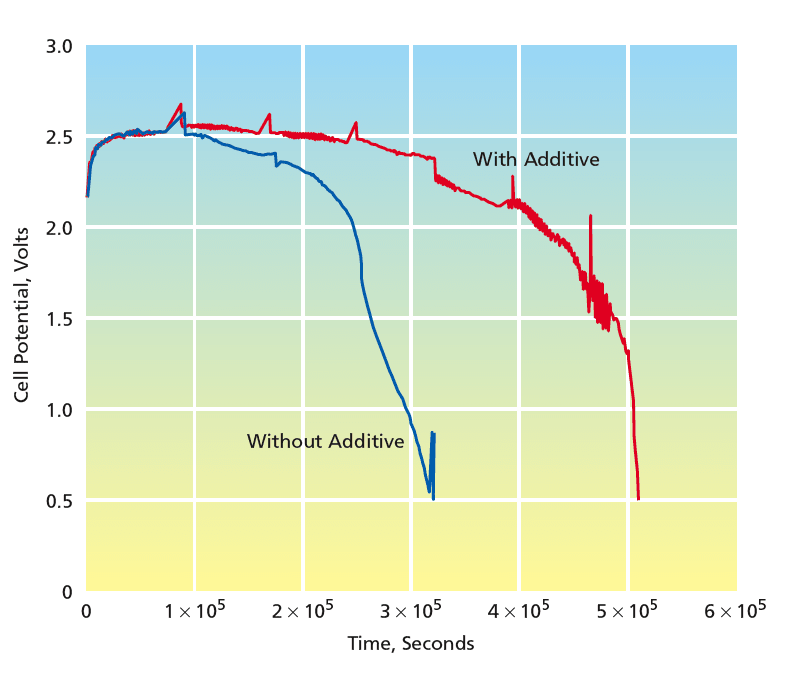
The promise of tris(hexafluoroisopropyl) borate as an additive was demonstrated in an experiment on two Li-(CF)n cells in standard commercial button-style packages. The cells were identical with one exception: the electrolyte solution in one cell contained 24 weight percent of tris(hexafluoroisopropyl) borate. The cells were tested at discharge currents of 1 mA. As shown in the figure, the cell containing the additive outperformed the one without the additive by a wide margin.
This work was done by Jay Whitacre and William West of Caltech for NASA's Jet Propulsion Laboratory.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to: Innovative Technology Assets Management
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109-8099
(818) 354-2240
E-mail:
Refer to NPO-42346, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Increasing Discharge Capacities of Li-(CF)n Cells
(reference NPO-42346) is currently available for download from the TSP library.
Don't have an account?
Overview
The document discusses advancements in lithium polycarbon monofluoride (Li-(CF)n) batteries, which are non-rechargeable batteries known for their high theoretical specific energy of 2180 Wh/kg and practical specific energies around 600 Wh/kg. Despite their advantages, these batteries are typically used in niche applications due to their low power density and inability to handle high drain currents, leading to their replacement by other battery systems like Li-MnO2.
To enhance the discharge capacity of Li-(CF)n batteries while maintaining high specific energy, the document explores the addition of anion receptors to the electrolyte. These receptors help reduce interfacial resistance at the cathode, improving rate capability and power density. The presence of anion receptors allows for better solubility of lithium fluoride (LiF) in the electrolyte, which is crucial during the discharge process. The document explains that the morphology of LiF deposits at the cathode can be significantly improved with the use of anion receptors, leading to larger, non-conformal grains that enhance electrolyte access and electronic conductivity.
Preliminary experimental results indicate that cells with anion receptors outperform control cells without additives. Specifically, at a discharge current of 1 mA, the cell with the anion receptor achieved a specific capacity of 598 mAh/g, compared to 342 mAh/g for the control cell. This demonstrates the potential of anion receptors to significantly enhance the performance of Li-(CF)n batteries.
The document also highlights the challenges associated with previous anion receptors, such as tris-(pentafluorophenyl) borane, which increased the viscosity of the electrolyte. In contrast, tris-(hexafluoroisopropyl) borate is noted for forming less viscous solutions while allowing for higher concentrations of LiF, thus improving conductivity.
Overall, the findings suggest that incorporating anion receptors into the electrolyte can lead to substantial improvements in the discharge capacity and overall performance of Li-(CF)n batteries, potentially opening new applications for these high specific energy cells. The research is part of NASA's efforts to develop technologies with broader scientific and commercial applications.

