Some progress has been reported in continuing research on the use of anion-receptor compounds as electrolyte additives to increase the sustainable rates of discharge and, hence, the discharge capacities, of lithium-poly(carbon monofluoride) [Li-(CF)n, where n >1] primary electrochemical power cells. Some results of this research at a prior stage were summarized in “Increasing Discharge Capacities of Li(CF)n Cells” (NPO-42346), NASA Tech Briefs, Vol. 32, No. 2 (February 2008), page 37. A major difference between the present and previously reported results is that now there is some additional focus on improving performance at temperatures from ambient down to as low as –40 °C.
- It renders LiF somewhat soluble in the non-aqueous electrolyte solution, thereby delaying precipitation until a high concentration of LiF in solution has been reached.
- When precipitation occurs, it promotes the formation of large LiF grains that do not conformally coat the cathode.
The net effect is to reduce the blockage caused by precipitation of LiF, thereby maintaining a greater degree of access of electrolyte to the cathode and greater electronic conductivity.
The anion-receptor compounds studied in this line of research have been fluorinated boron-based compounds. The specific compound mentioned in the cited prior article, in which there was no special focus on low-temperature performance, was tris(hexafluoroisopropyl) borate. The anion-receptor compound used in the more-recent research reported here — tris(2,2,2-trifluoroethyl) borate — was selected because of an expectation that it would reduce the viscosity of the electrolyte, thereby increasing the low-temperature conductivity and, consequently, increasing the low-temperature discharge-rate capability. One complicating observation made in this research was that tris(2,2,2-trifluoroethyl) borate does not improve the low-temperature performance of a cell containing a fully fluorinated (CF)n cathode, but does improve the low-temperature performance of a cell containing a sub- fluorinated (CF)n cathode — that is, a cathode made of (CFx)n [where x<1].
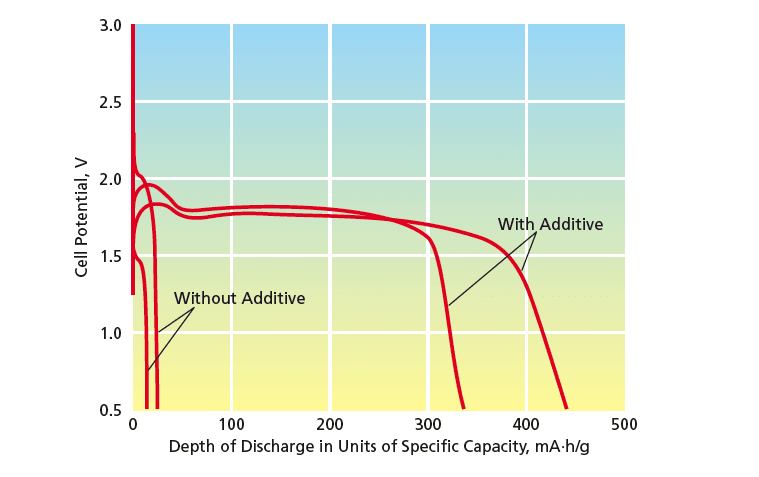
The improvement in low-temperature performance can be considerable. For example, in one set of tests at a temperature of –40 °C, a pair of cells that did not contain the present anion-receptor additive and another pair of cells that did contain this additive were discharged at a current of C/2.5 (where C is the magnitude of the current, integrated for one hour, that would amount to the nominal charge capacity of a cell). The results of the tests (see figure) showed that the cells containing the additive performed much better than did the cells without the additive.
This work was done by William West and Jay Whitacre of Caltech for NASA’s Jet Propulsion Laboratory.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to:
Innovative Technology Assets Management
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109-8099
(818) 354-2240
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Refer to NPO-43579, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Additive for Low-Temperature Operation of Li-(CF)n Cells
(reference NPO-43579) is currently available for download from the TSP library.
Don't have an account?
Overview
The document discusses advancements in lithium polycarbon monofluoride (Li-CFx) batteries, specifically focusing on improving their performance at low temperatures. These batteries are known for their high specific energy but face limitations in discharge rates, particularly when temperatures drop. The primary challenge is that while these cells can sustain long discharge periods, they struggle with high current outputs, and this issue worsens at lower temperatures.
To address this problem, the research presented in the document highlights the incorporation of anion receptors into the conventional Li-CFx battery electrolytes. The key finding is that adding tris-(2,2,2-trifluoroethyl) borate, a low molecular weight and low viscosity compound, significantly enhances the rate capability of these batteries at reduced temperatures. Experimental results indicate that cells with this additive can achieve nearly 50% of their room temperature capacity even at -40 °C, a substantial improvement compared to cells without the additive, which were nearly nonfunctional at that temperature.
The document emphasizes the novelty of these findings, noting that the enhanced performance is particularly pronounced with sub-fluorinated CFx materials (where x<1). However, the exact mechanism behind this improvement remains unclear, indicating a need for further research to fully understand the interactions at play. Additionally, the document points out that the parameter trade space for optimizing the molarity and type of anion receptor or salt is still unmapped, suggesting that further enhancements could be achieved through systematic optimization.
In summary, the research demonstrates a promising approach to overcoming the limitations of Li-CFx batteries in low-temperature environments by utilizing specific electrolyte additives. This work not only contributes to the field of battery technology but also has potential implications for various applications, particularly in aerospace, where reliable energy storage at low temperatures is critical. The findings are documented in several references, showcasing a collaborative effort in advancing battery technology for broader scientific and commercial applications.

