“Our lithium-air battery design represents a revolution in the battery community,” said Amin Salehi-Khojin, assistant professor of mechanical and industrial engineering at the University of Illinois at Chicago.
Salehi-Khojin and fellow researchers at UIC and Argonne National Laboratory have designed an electrochemical cell that works in a natural air environment and still functions after a record-breaking 750 charge/discharge cycles.
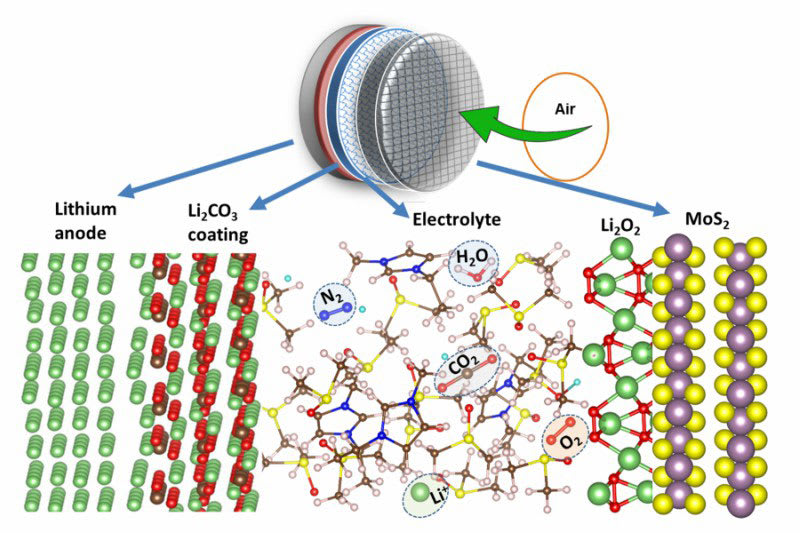
A lithium-air battery combines oxygen from the air with lithium present in the anode. The mix produces lithium peroxide during the discharge phase – and a breakdown of lithium and oxygen components in the charge phase.

Lithium-air batteries are believed to have the capacity to hold up to five times more energy than the same lithium-ion batteries powering today’s phones, laptops, and electric vehicles.
Early “lithium-air” ideas, however, have frequently failed.
When lithium ions combine with carbon dioxide and water vapor in the air, the result has often been byproducts that gum up the cathode.
To prevent the buildup and allow the battery to operate in a natural-air environment, the UIC and Argonne researchers coated the lithium anode with a thin layer of lithium carbonate. The coating selectively allows lithium ions from the anode to enter the electrolyte – while preventing unwanted compounds from reaching the anode.
In experimental designs of lithium-air batteries, oxygen enters the electrolyte through a carbon-based spongy lattice structure.
Salehi-Khojin and his colleagues coated the lattice structure with a molybdenum disulfate catalyst. The unique hybrid electrolyte – made of ionic liquid and dimethyl sulfoxide, a common component of battery electrolytes – helped to facilitate lithium-oxygen reactions, minimize lithium reactions with other elements in the air, and boost the efficiency of the battery.
Our readers, however, had questions. What if the battery is overheated? Can the battery explode? Are there safety concerns? How does a lithium-air battery stack up against a lithium-ion battery? Larry Curtiss, Argonne Distinguished Fellow and co-principal author of the study, took the time to provide answers to our audience. See the responses below.
What is the charging rate?
Curtiss: The charging rate at this time would be similar to a Li-ion battery. It could be increased with further research.
How does your new design outpower Li-ion batteries?
By using chemical bonds between Li and oxygen, the batteries can store much more energy, because the bonds are more dense than the intercalation interactions between Li and the metal oxide layers used in Li-ion batteries.
What is the potential for flammability or explosion (if punctured, overheated, overcharged, etc.)?
Curtiss: One component of the Li-air battery we published is the lithium anode. It is known that it could cause an explosion. Many scientists are working on the problem of the safety of the lithium anode and will probably take much effort to get it safe. However, it is worth mentioning that we have protected the surface of the Li anode with an electrically insulator but ionically conductive material in order to prevent any explosion due to battery short circuit between anode and cathode. This prevents overheating of the battery too.
What are the failure modes expected to be?
Curtiss: This will be investigated during scale up.
Any other safety concerns? The energy density of standard Li-Ion batteries is equal to that of TNT. Your batteries have 5x that energy density. Although in theory they limit the undesired reactions by these thin coatings, there are other potential contaminants in air (CO2 N2, etc.) Could some of these become corrosive and break down the layers? Could internal short circuits develop reversing a single cell leading to fire?
Curtiss: The battery has been developed at only the lab scale where it has not exhibited any of these safety issues during the tests. The next step would be to scale these small cells up to battery packs, test them, and find out what the safety issues are and address them. However, we believe that the overheating due to cell reversing would not occur in our system due to robust protection layer of the Li anode surface.
What do you think? Do you have more questions about lithium-air batteries? Share them below.