Lithium metal may potentially increase the energy density in rechargeable batteries beyond what is currently achieved by commercial lithium-ion batteries. The key to improving density lies in developing a powerful, thin, solid electrolyte. Solid polymer electrolytes are flexible and low-cost but have low conductivity while ceramic-based electrolytes offer better conductivity but are too brittle to process.
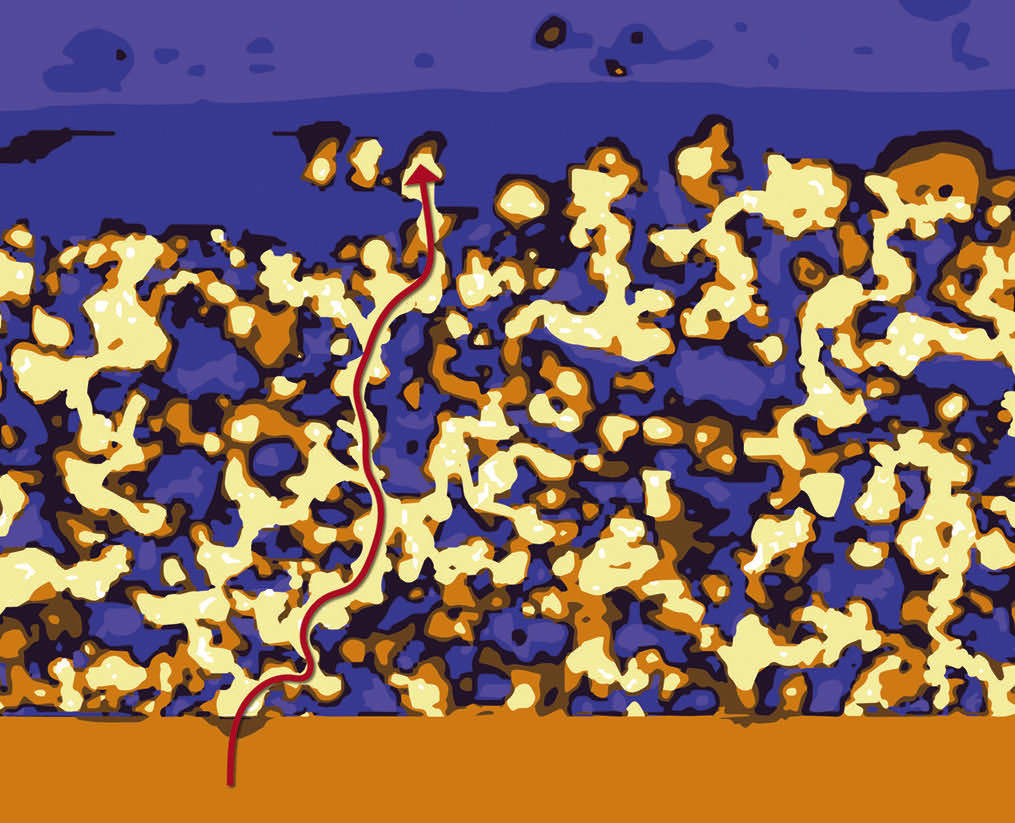
Researchers have developed a thin-film, highly conductive, solid-state electrolyte made of a polymer and ceramic-based composite for lithium metal batteries. The electrolyte’s novel design is a three-dimensional interconnected structure that can provide mechanical robustness and high lithium ionic conductivity at room temperature.
The film was fabricated by first forming a doped-lithium aluminum titanium phosphate ceramic thin film with thickness of ~25 μm by aqueous spray coating, a scalable process. The film is partially sintered to form a three-dimensionally interconnected structure with a dense backbone. It is then backfilled with a cross-linkable poly(ethylene oxide) (PEO)-based polymer electrolyte.
For more information, contact Jennifer J. Burke at