While scientists have made some progress with sodium-ion batteries, hurdles arise largely because of their low energy density: they have shorter battery-run times relative to their size. High power density, the rate at which energy can be released, also factors into their performance. Achieving high energy density and high power density simultaneously has been an ongoing challenge for alternative batteries.
But the cathode material put forward by Princeton University’s Dincă Group, a layered organic solid called bis-tetraaminobenzoquinone (TAQ), outperforms traditional lithium-ion cathodes in both energy and power densities in a technology that is truly scalable.
Their research has potential for large-scale energy storage applications like data centers, power grids, and commercial-scale renewable energy systems, in addition to electric vehicles.
“Everyone understands the challenges that come with having limited resources for something as important as batteries, and lithium certainly qualifies as ‘limited’ in a number of ways,” said Mircea Dincă, the Alexander Stewart 1886 Professor of Chemistry. “It’s always better to have a diversified portfolio for these materials. Sodium is literally everywhere. For us, going after batteries that are made with really abundant resources like the organic matter and seawater is among our greatest research dreams.

“Energy density is something on a lot of people’s minds because you can equate it with how much juice you get in a battery. The more energy density you have, the farther your car goes before you have to recharge it. We’ve answered quite emphatically that the new material we developed has the largest energy density, certainly on a per kilogram basis, and competes with the best materials out there even on a volumetric basis.
“Being on the front lines of developing a truly sustainable and cost-effective sodium ion cathode or battery is truly exciting.”
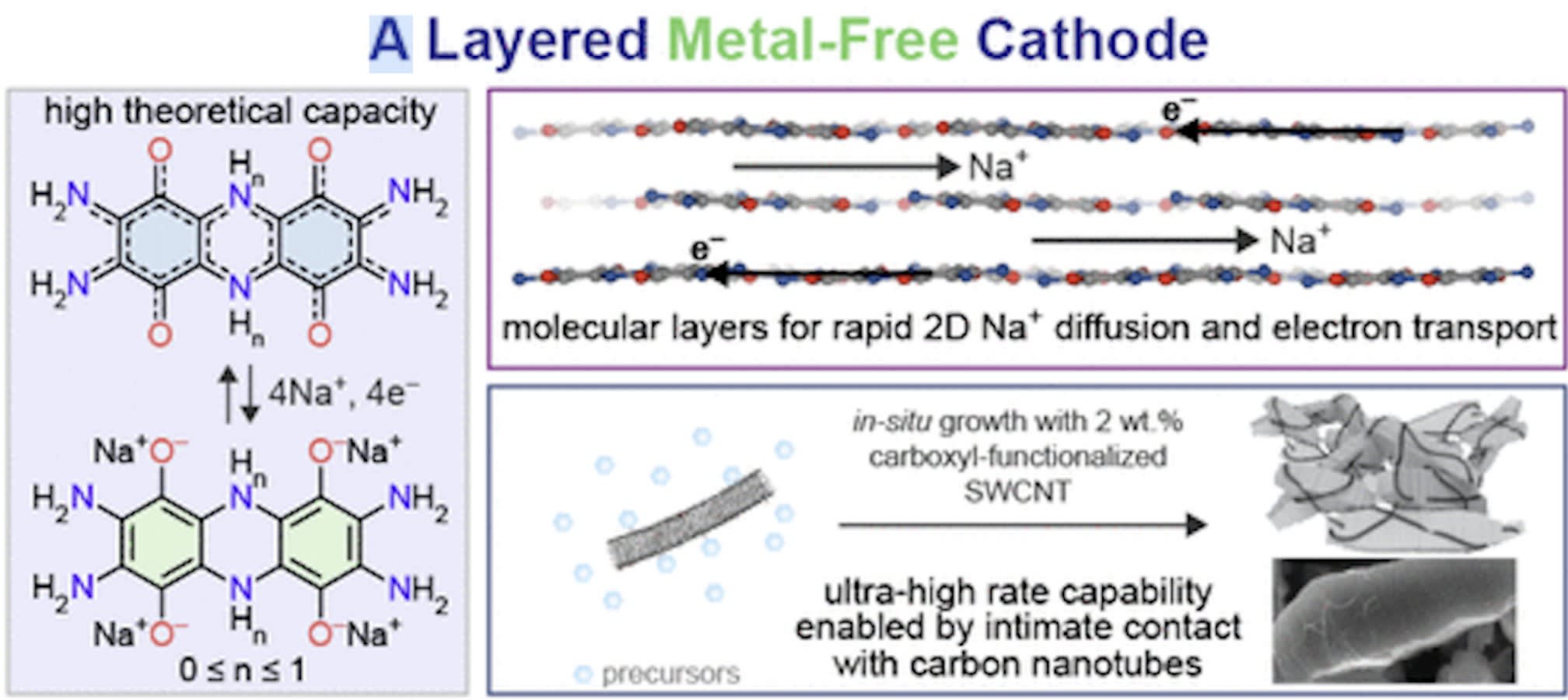
The lab underscored the advantages of TAQ a year ago when they first reported on its utility for making lithium-ion batteries in ACS Central Science. Researchers simply continued investigating its potential, particularly when they found TAQ to be completely insoluble and highly conductive, two key technical advantages for an organic cathode material. A cathode is an essential component of all polarized devices.
So, they endeavored to construct an organic, sodium-ion battery using the same material, TAQ. The process took about a year, as researchers had to adapt several design principles that could not be ported over from lithium-ion technology.
In the end, the results exceeded their expectations. Their cathode’s performance is close to a benchmark known as the theoretical maximum capacity.
“The binder we chose, carbon nanotubes, facilitates the mixing of TAQ crystallites and carbon black particles, leading to a homogeneous electrode,” said Dincă Group Ph.D. and first author on the paper, Tianyang Chen. “The carbon nanotubes closely wrap around TAQ crystallites and interconnect them. Both of these factors promote electron transport within the electrode bulk, enabling an almost 100% active material utilization, which leads to almost theoretical maximum capacity.
“The use of carbon nanotubes considerably improves the rate performance of the battery, which means that the battery can store the same amount of energy within a much shorter charging time, or can store much more energy within the same charging time.”
Chen said TAQ’s benefit as a cathode material also include its stability against air and moisture, long lifespan, ability to withstand high temperatures, and environmental sustainability.
Here is an exclusive Tech Briefs interview, edited for length and clarity, with Chen.
Tech Briefs: What was the biggest technical challenge you faced while developing this cathode?

Chen: The biggest technical challenge was to enable high energy density and high discharge rate capability of the cathode while maintaining a practical level of active material content, 90 percent by weight.
Tech Briefs: What has changed since your lab first reported on TAQ a year ago? How has your work advanced?
Chen: We have demonstrated a new strategy of wrapping TAQ particles with carbon nanotubes through an in-situ growth method, which significantly enhances the rate performance of the cathode. This method relies on the hydrogen bonding and covalent bonding between TAQ and the carboxyl functional groups on the surface of carbon nanotubes.
Tech Briefs: Can you explain in simple terms how TAQ works in this case please?
Chen: We paired the TAQ cathode with either sodium metal anode or pre-sodiated hard carbon anode to fabricate coin cells with NaPF6 in DME/diglyme (vol/vol = 1:1) as the electrolyte. While discharging, one TAQ molecule can accept four sodium ions and four electrons, exhibiting a theoretical capacity of 355 mAh/g.
Tech Briefs: Do you have any plans for further research/work/etc.? If not, what are your next steps?
Chen: The Dincă lab is working on developing multi-valent metal batteries, which would showcase new potential applications for this material, which can be fitted into a variety of different battery systems to meet different energy and power needs. Prof. Dincă and Dr. Harish Banda (my collaborator) have launched a startup, DAQUS, based on our techniques. I am sure that much more progress on the commercialization of our technology will come in the near future.
Tech Briefs: Is there anything else you’d like to add that I didn’t touch upon?
Chen: I want to highlight that TAQ cathodes can compete with LiFePO4-based lithium batteries (one of the leading Li-ion battery technologies) in terms of both energy density and power density.
Tech Briefs: Do you have any advice for researchers aiming to bring their ideas to fruition?
Chen: Knowing the most urgent questions in a certain field is important for setting up goals for research. With a clear goal in mind, it can make research more efficient. In addition, always be curious and pay attention to details.

