Dr. James Rees is spending the time in his lab testing sensors made from bacteria. The living sensors are, in fact, microbes growing on an electrode.
Rees, a postdoctoral research associate at Rensselaer Polytechnic Institute , is working with a bacteria called Shewanella oneidensis, which absorbs metals and produces a resulting useful material for electronics.
The bacteria's byproduct, a compound known as molybdenum disulfide, transfers electrons easily, like graphene, and acts as a kind of nanowire.
If the RPI researchers can control the ability of bacteria to produce electronic materials — a difficult task, no doubt — the team hopes to create a new class of nutrient sensors that can be deployed on bodies of water.
By measuring the electrochemical behavior of a lake, stream, or aquatic ecosystem, Rees wants to measure biological and chemical data in real time.
"It could improve our ability to detect pollutants in these environments as well as some of the secondary effects of those pollutants, such as harmful algal blooms," Rees told Tech Briefs.

Rees led the sensor research project, a collaboration between Rensselaer, IBM Research, and The FUND for Lake George , a nonprofit that is pioneering a new model for environmental monitoring and prediction. ( Read the team's research .)
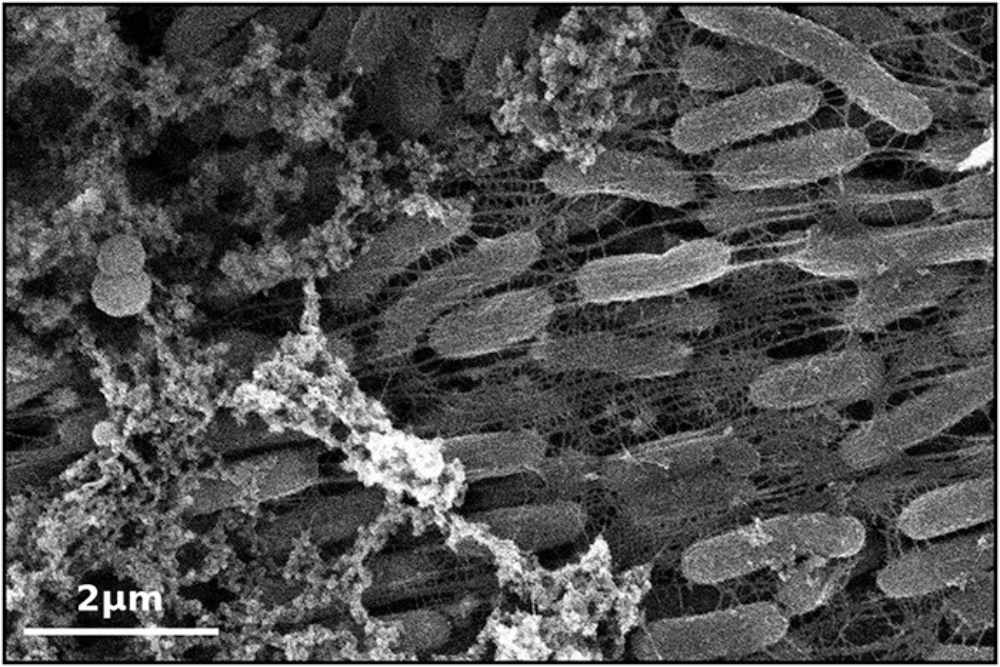
The sensor development begins by coaxing the bacteria to create the inorganic compound composed of molybdenum and sulfur.
After the team provides molybdenum trioxide minerals to the Shewanella, the bacteria "reaches out" and reduces the substance to molybdenum disulfide (shown in the top image).
The bacteria's byproduct of molybdenum disulfide, a “2D material,” has a crystal structure consisting of atom-thin sheets weakly bonded together. The loose arrangement gives the material special electronic and photonic properties, including a high level of tunability, that are of great interest to engineers.
In a short Q&A below, Rees tells Tech Briefs how his team is trying to harness and control the bacteria — and its potential for electronics.
Tech Briefs: What does a bacteria sensor look like then? What are its components?
Dr. James Rees: When we say “living sensor” or “bacterial sensor” in the context of our research, we are talking specifically about microbes that are growing on an electrode or conductive matrix in an aquatic environment – the environment to be tested. Right now we are testing proofs of concept in enclosed systems in our lab. The Sawyer research group , which I’m a part of, has experience designing inorganic/organic nanomaterial-based electronic devices, and we are drawing upon that knowledge to figure out how to build the optimal electrode or matrix to facilitate charge transfer from the bacteria. This charge transfer is what we measure in order to do biosensing.
Tech Briefs: What are the most exciting applications that you envision for living sensors?
Dr. James Rees: Right now we are primarily interested in the environmental applications for these living sensors. Environmental scientists and ecologists try to understand aquatic ecosystems by measuring microbial activity and nutrient levels. Chemical sensors generally contain test solutions that must be periodically replaced, and studying microbial activity usually involves taking a sample back to the lab for analysis. We believe that by measuring the electrochemical behavior of an aquatic ecosystem, we can collect both biological and chemical data in real time. This would be tremendously useful for understanding what’s happening in lakes, streams, and other aquatic environments. It could improve our ability to detect pollutants in these environments as well as some of the secondary effects of those pollutants, such as harmful algal blooms.
Tech Briefs: What did your most recent test demonstrate?
Dr. James Rees: Our lab’s bio-nanotechnology research so far has had a two-fold approach. First, we are interested in asking, “What types of electronic and photonic materials can we make with bacteria, and how can we better control the synthesis process?” Our recent paper in AVS Biointerphases reflects this line of thinking. Our future experiments will attempt to control variables such as what crystal structure the generated materials have, where they appear, and how large the crystals are. From an electronics fabrication perspective, it’s desirable to to control all these variables.
Tech Briefs: What's next?
Dr. James Rees: The second half of our lab’s approach is, once again, harnessing bacteria for biosensing. In our preliminary results we have collected data showing the distinct current signature of Shewanella bacteria, and can see evidence of how the bacteria affect the chemistry of the surrounding water. From here we will focus on gauging how bacteria respond to some common environmental pollutants, especially those that are normally difficult and expensive to quantify in real time.
Tech Briefs: How easily are you able to get bacteria to produce molybdenum disulfide?
Dr. James Rees: This depends on how you define “easy.” In one sense, bacteria are actually able to produce these materials more easily than conventional methods. These types of materials are often produced using high-temperature vapors and chemical solvents.
Bacteria like Shewanella oneidensis can make the same materials at room temperature in a liquid medium consisting essentially of salts, vitamins, and a few nutrients. This is very promising in terms of sustainable materials synthesis. However, the tricky part is that biological systems are much more difficult to control and the materials they produce can be more varied in terms of structure and composition than those produced by purely chemical systems. This is one way that working with microbiology makes nanomaterial synthesis more difficult – microbial cultures are complex, varied environments and their products are varied.
However, this difficulty is also an added strength: microbial processes can potentially produce a much wider array of nanomaterial products than their nonbiological counterparts. It’s a matter of learning how to better control the process, and that’s where a lot of our future research lies.

Tech Briefs: What inspired you to use this type of design?
Dr. James Rees: I’ve been fascinated by the potential for biological electronics since early in my grad school days when we covered organic semiconductors in one of my courses. When I learned about Shewanella oneidensis, I realized that some bacteria are, in a sense, organic semiconductors with the ability to assemble inorganic semiconductors. Nanomaterials synthesis and biosensing are two areas where other scientists have already identified tremendous potential for using the abilities of electroactive bacteria, and my goal is to help us move closer to those applications.
Tech Briefs: Are fellow researchers surprised at this kind of design?
Dr. James Rees: We absolutely get a lot of questions about our work. It’s somewhere in between electrical engineering, microbiology, and materials science. While doing my PhD, I was co-advised by an electrical engineer (Shayla Sawyer) and a microbiologist (Yuri Gorby). The three of us had to work together to translate the concepts from these different fields, and in the process have found ourselves in kind of a new area between disciplines. It’s sometimes hard to relate to researchers who are squarely in just one of these fields. However, I’ve met scientists at conferences who are doing similar interdisciplinary work and had some terrific conversations with them. I think this area of research will grow, and I’m very excited to see where it goes.
What do you think? Share your questions and comments below.