Maximizing the natural corrosion resistance of parts and components machined from stainless steels. It can make the difference between satisfactory performance and premature failure. Incorrectly performed, passivation can actually induce corrosion.
The process of passivation is a post-fabrication method of maximizing the inherent corrosion resistance of the stainless alloy from which the workpiece was produced. It is not a treatment to descale or coat the piece. While there is no consensus on how the process works, a protective oxide film occurs naturally on the surface of passive stainless as the result of exposure to oxygen in the atmosphere. Any clean, freshly machined, polished, or pickled stainless steel part automatically acquires this protective film. Under ideal conditions, it covers all surfaces of the part. The film is extremely thin — just 0.0000001" thick.
During the machining process, contaminants such as shop dirt or particles of iron from cutting tools can be transferred to the surface of the stainless steel parts. Under certain conditions, this contamination can produce a thin coating of rust on the part. Sometimes the crevice where a particle of tool steel is embedded in the surface of the finished part can propagate a corrosive attack on the part itself. Similarly, small particles of iron-containing shop dirt may adhere to the part surface. If not removed, these particles can reduce the effectiveness of the original protective film.
Exposed sulfides also can be a problem. Sulfides improve an alloy's ability to form chips that break away cleanly from a cutting tool. Unless the part is properly passivated, sulfides can act as initiation sites for corrosion on the surface of the fabricated part.
In both cases, passivation is needed to maximize the natural corrosion resistance of the stainless steel. It can remove both surface contamination and sulfides. A two-step procedure — cleaning, followed by an acid bath — provides the best possible corrosion resistance.
Cleaning should always come first. Machining chips or other shop dirt can be wiped carefully from the part. A commercial degreaser or cleanser can remove machining oils or coolants. Grinding or acid pickling can remove thermal oxides and other foreign matter.
The importance of cleaning should not be underestimated. Sometimes a machine operator might skip the process, assuming that by immersing the grease-laden part in an acid bath, it will be both cleaned and passivated. Instead, however, the contaminating grease reacts with the acid to form gas bubbles that collect on the surface of the workpiece and interfere with passivation. Even worse, contamination of the passivating solution, sometimes with high levels of chlorides, can cause "flash attack," producing a heavily etched or darkened surface.
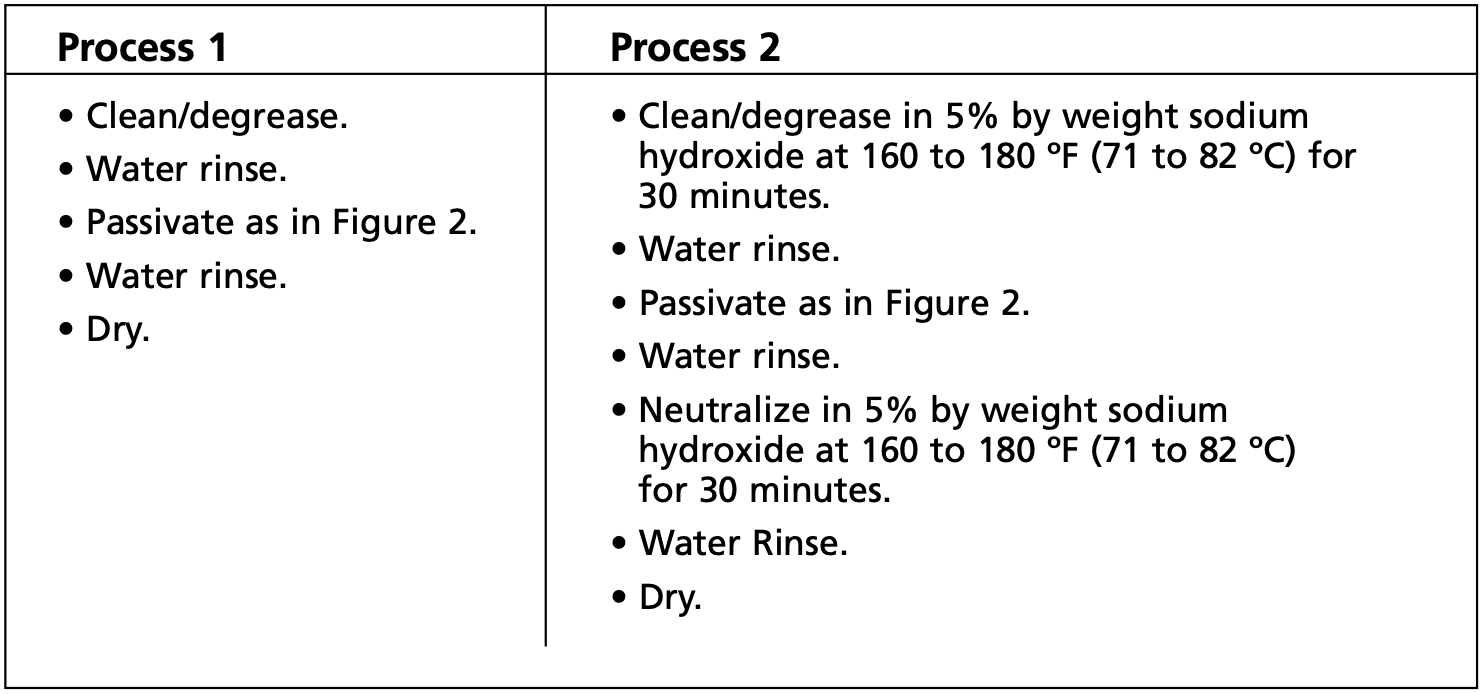
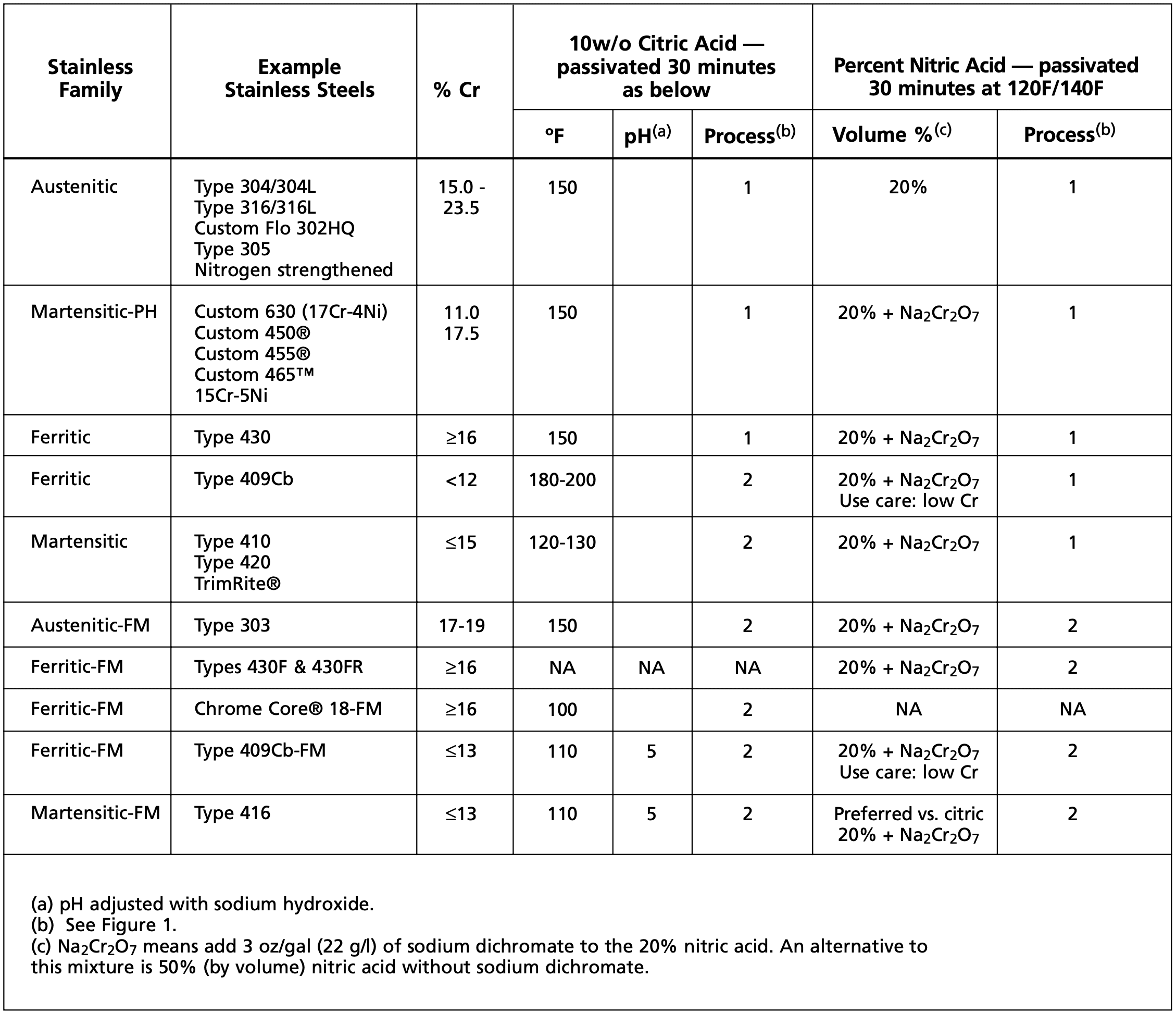
After cleaning, the part is ready for immersion in a passivating acid bath (see Figure 1). Any one of three media can be used: nitric acid, nitric acid with sodium dichromate, or citric acid. Which bath is used depends on the grade of stainless steel and prescribed acceptance criteria.
Citric acid passivation has become increasingly popular with fabricators who want to avoid the use of mineral acids or solutions containing sodium dichromate, along with the disposal problems and safety concerns associated with their use (see Figure 2). Citric acid is on the GRAS (Generally Regarded as Safe) list compiled by the U.S. Food and Drug Administration (FDA) as a material that is safe for humans to handle.
Tests are often performed to evaluate the surface of passivated parts. The 400 series, martensitic precipitation-hardening and free-machining stainless steels are best evaluated in a cabinet capable of maintaining 100% humidity (samples wet) at 95 °F (35 °C) for 24 hours. Austenitic non-free-machining stainless grades also may be evaluated by means of a humidity test. A faster method is available using a solution from ASTM A380, "Standard Practice for Cleaning, Descaling, and Passivation of Stainless Steel Parts, Equipment, and Systems." This test consists of swabbing the part with a copper sulfate/sulfuric acid solution, maintaining wetness for six minutes, and observing whether there is any plating of copper.
It is important that the test method be matched to the grade under evaluation. A test that is too severe will reject perfectly good material, while one that is too lenient will allow unsatisfactory parts to be accepted.
This article was written by Terry A. DeBold and Ted Kosa, staff specialists in stainless alloy R&D at Carpenter Technology Corp. For further information, contact Terry DeBold at

