A number of cellphone fires in 2016 brought attention to lithium-ion batteries, a technology that helped advance modern portable electronics but has been plagued by safety concerns. As interest in electric vehicles increases, so does the need for improved rechargeable battery technology that can safely and reliably power cars, autonomous vehicles, robotics, and other next-generation devices.
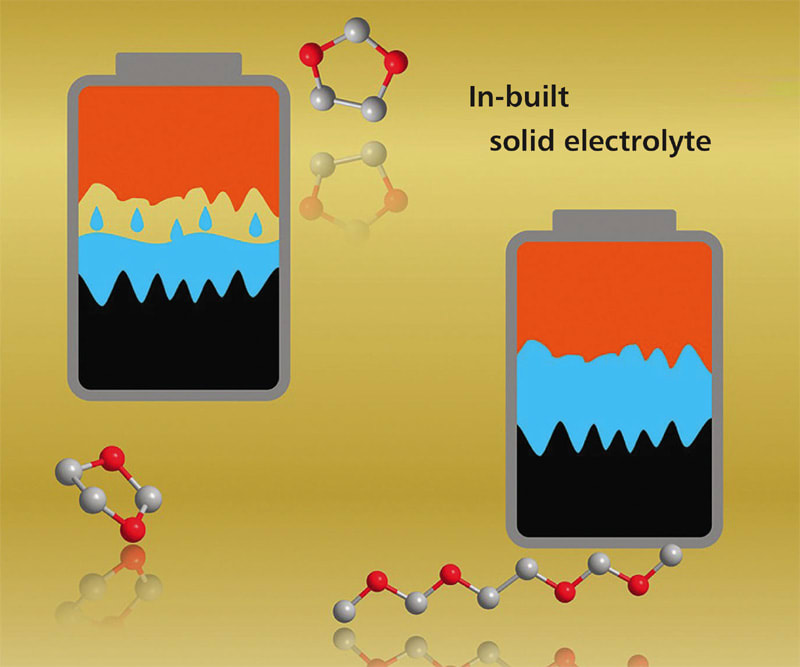
Solid-state batteries are inherently safer and more energy-dense than today’s lithium-ion batteries, which rely on flammable liquid electrolytes for fast transfer of chemical energy stored in molecular bonds to electricity. By starting with liquid electrolytes and then transforming them into solid polymers inside the electrochemical cell, researchers have taken advantage of both liquid and solid properties to overcome key limitations in current battery designs.
The key insight is the introduction of special molecules capable of initiating polymerization inside the electrochemical cell without compromising other functions of the cell. If the electrolyte is a cyclic ether, the initiator can be designed to rip open the ring, producing reactive monomer strands that bond together to create long chain-like molecules with essentially the same chemistry as the ether. This now-solid polymer retains the tight connections at the metal interfaces.
Beyond their relevance for improving battery safety, solid-state electrolytes are also beneficial for enabling next-generation batteries that utilize metals, including lithium and aluminum, as anodes for achieving far more energy storage than is possible in today’s state-of-the-art battery technology. In this context, the solid-state electrolyte prevents the metal from forming dendrites, a phenomenon that can short-circuit a battery and lead to overheating and failure.
Despite the perceived advantages of solid-state batteries, industry attempts to produce them on a large scale have encountered setbacks. Manufacturing costs are high, and the poor interfacial properties of previous designs present significant technical hurdles. A solid-state system also circumvents the need for battery cooling by providing stability to thermal changes.
The new in-situ strategy for creating solid polymer electrolytes shows promise for extending cycle life and recharging capabilities of high-energy-density rechargeable metal batteries.
For more information, contact Jeff Tyson at