Batteries and combustion engines each have distinctive benefits and limitations. Batteries have simple construction and operate silently; however, their energy density (i.e., the energy per unit volume) is poor, and lithium-ion batteries are potential fire hazards. The energy densities of combustion engines are higher than those of batteries, but combustion engines are relatively loud and emit toxic gases.
A power system was developed that may provide the advantages of simple construction, silent operation, and high energy density. The energy system employs an aluminum-based fuel that is potentially safer, more reliable, and easier to refuel than alternatives. Additionally, an aluminum-fueled power system is simpler to start up and shut down than are gasoline engines, and the system operates in extreme environments such as beneath the sea.
The basic chemistry of aluminum as a fuel relies on a reaction with water to generate hydrogen and heat according to the following:
2Al + 6H2 → 2Al(OH)3 + 3H2O + Q (Heat)
This reaction releases approximately 84 MJ/L of energy (almost evenly split between heat energy and potential energy in the form of hydrogen), which is more than twice the volumetric energy density of diesel fuel, and more than 3.5 times that of lithium.
Reacting aluminum with water, however, is challenging because a very stable oxide layer that forms on the surface of raw aluminum typically inhibits a reaction when the aluminum is exposed to air or water. This thin, but impervious layer is the reason that aluminum soda cans do not react with the beverage within. Because penetrating or inhibiting this oxide layer is key to unlocking the energy stored in aluminum, researchers have investigated a number of methods to remove or disrupt the oxide layer on aluminum, including applying strong acids, heating the aluminum, and alloying the aluminum with other metals.
The concept for inhibiting the oxide layer in this work is surface-treating aluminum with a thin eutectic (i.e., a mixture of two or more compounds) layer of gallium, indium, and tin. This treatment results in a fuel that consists of approximately 98% aluminum and 2% gallium, indium, and tin. This fuel reacts with water over a wide range of temperatures. But more importantly, this safe, energy-dense fuel can be stored without degrading over time.
The hydrogen released by the aluminum fuel can be used in a relatively simple system to generate electrical power with a commercial fuel cell. In this concept, water is metered into a reaction chamber containing fuel, and hydrogen from the aluminum-water reaction is fed into a fuel cell. If the system operates in air, then oxygen gas, also required by the fuel cell, can be extracted from the surrounding air. If the system operates below the ocean’s surface, then it needs a separate oxygen source such as compressed or liquid oxygen. Alternatives to oxygen gas include chemical compounds such as sodium chlorate (which disassociates into sodium chloride and oxygen with heat) or hydrogen peroxide (which disassociates into water and oxygen through a catalytic reaction with silver).
Several prototype systems have been designed, built, and tested, demonstrating the scalability of an aluminum-water power system in diverse applications. These prototypes produced power ranging from 30W to 3kW. The smallest prototype outputs 30W of power and is designed to fit in a backpack for a dismounted field soldier or hiker.
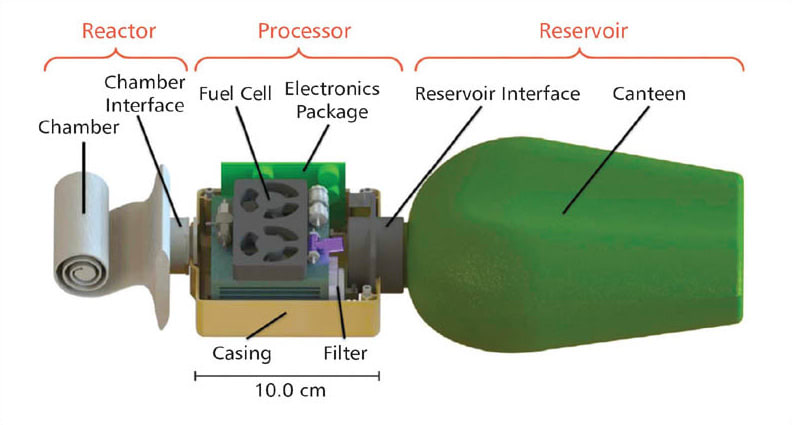
The Emergency Power Pack consists of a disposable reactor chamber containing the aluminum fuel, a processor with a fuel cell, and a water reservoir (see figure). The prototype pack weighs 734 grams and was designed to output 30W for up to 10 hours.
A larger 200W benchtop system was developed for powering a mid-sized unmanned undersea vehicle (UUV). This UUV power system was built before the aluminum fuel was optimized, and employed a different approach to overcome the aluminum oxide layer. Rather than using a eutectic coating on the aluminum, the 200W system incorporates a liquid gallium reservoir into which raw aluminum is introduced. The aluminum dissolves into the gallium bath, and when water is introduced, the aluminum reacts with the water. Hydrogen is produced from this reaction, but because the fuel cell also requires oxygen and must operate below the ocean surface, a separate oxygen system that uses sodium chlorate was developed.
This system, which ingests seawater from the environment to drive the reaction, was designed to power a mid-sized UUV for 30 days at three knots, a tenfold increase in total energy over lithium-ion batteries.
For more information, contact Nicholas Pulsone, Advanced Undersea Systems & Technology Group, at

