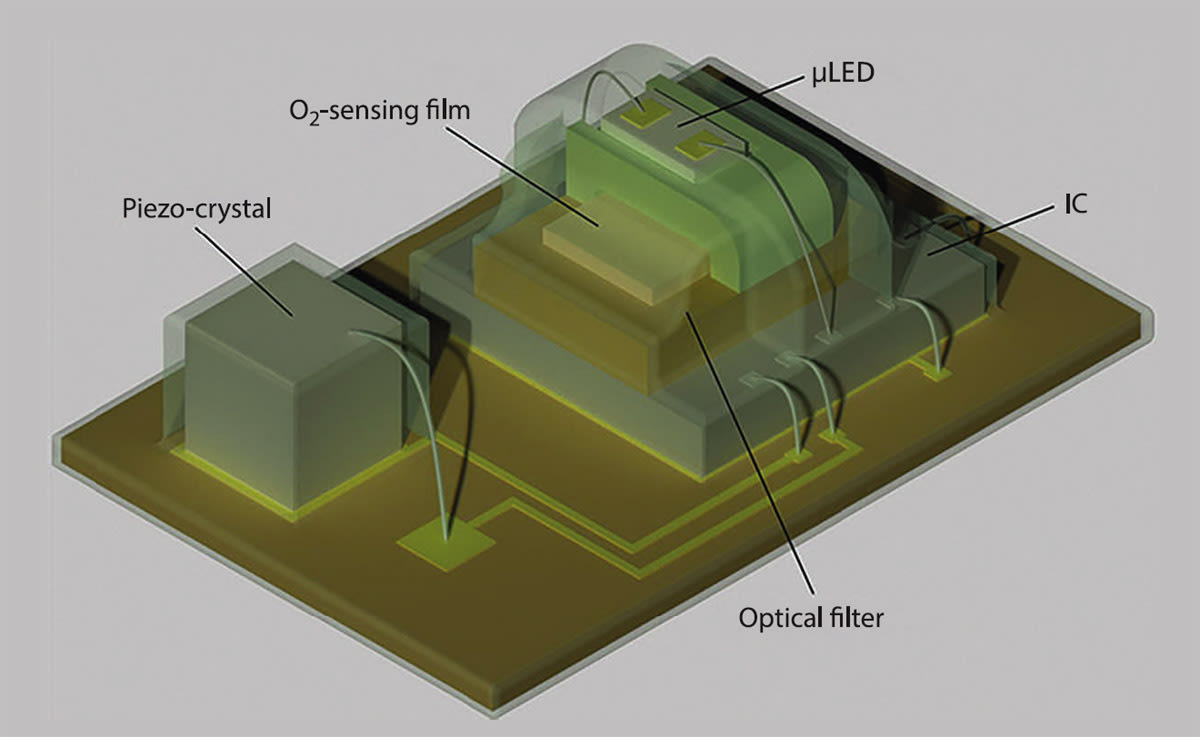
Engineers have created a tiny wireless implant that can provide real-time measurements of tissue oxygen levels deep underneath the skin. The device, which is smaller than the average ladybug and powered by ultrasound waves, paves the way for the creation of a variety of miniaturized sensors that could track key biochemical markers in the body such as pH or carbon dioxide. These sensors could one day provide doctors with minimally invasive methods for monitoring the biochemistry inside functioning organs and tissues.
Oxygen is a key component to cells’ ability to harness energy from the food that we eat and nearly all tissues in the body require a steady supply in order to survive. Most methods for measuring tissue oxygenation can only provide information about what is happening near the surface of the body. That is because these methods rely on electromagnetic waves, such as infrared light, which can only penetrate a few centimeters into skin or organ tissue. While there are types of magnetic resonance imaging that can provide information about deep tissue oxygenation, they require long scanning times and so are unable to provide data in real time.
Ultrasonic waves, which are a form of sound too high in frequency to be detected by the human ear, can travel harmlessly through the body at much longer distances than electromagnetic waves and are already the basis of ultrasound imaging technology in medicine.
Incorporating the oxygen sensor involved integrating both an LED light source and an optical detector into the tiny device as well as designing a more complicated set of electronic controls to operate and read out the sensor. This type of oxygen sensor differs from the pulse oximeters that are used to measure oxygen saturation in the blood. While pulse oximeters measure the proportion of hemoglobin in the blood that is oxygenated, the new device is able to directly measure the amount of oxygen in tissue.

One potential application of the device is to monitor organ transplants because in the months after organ transplantation, vascular complications can occur and these complications may lead to graft dysfunction. It could be used to measure tumor hypoxia as well, which can help doctors guide cancer radiation therapy.
In premature infants, supplemental oxygen may be required but doctors don’t have a reliable tissue readout of oxygen concentration. Further miniaturized versions of this device could help better manage oxygen exposure in preterm infants in the intensive care nursery setting and help minimize some of the negative consequences of excessive oxygen exposure such as retinopathy of prematurity or chronic lung disease.
The technology could be further improved by housing the sensor so that it could survive long term in the body. Further miniaturizing the device would also simplify the implantation process, which currently requires surgery. In addition, the optical platform in the sensor could be readily adapted to measure other biochemistry in the body. By just changing the platform that was built for the oxygen sensor, the device can be modified to measure, for example, pH, reactive oxygen species, glucose, or carbon dioxide. If the packaging could be made smaller, the device could be injected into the body with a needle, or through laparoscopic surgery, making the implantation even easier.
For more information, contact Kara Manke at