Integrated microbatteries have been proposed to satisfy an anticipated need for long-life, low-rate primary batteries, having volumes less than 1 mm3, to power electronic circuitry in implantable medical devices. In one contemplated application, such a battery would be incorporated into a tubular hearing-aid device to be installed against an eardrum. This device is based on existing tube structures that have already been approved by the FDA for use in human ears.
This work was done by Jay Whitacre and William West of Caltech for NASA's Jet Propulsion Laboratory.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to: Innovative Technology Assets Management
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109-8099
E-mail:
Refer to NPO-42287, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Integrated Microbatteries for Implantable Medical Devices
(reference NPO-42287) is currently available for download from the TSP library.
Don't have an account?
Overview
The document is a Technical Support Package from NASA's Jet Propulsion Laboratory (JPL) detailing the development of Integrated Microbatteries for Implantable Medical Devices, specifically focusing on a primary micro battery designed for hearing aids. The primary challenge addressed is the need for a high energy density battery that occupies minimal volume while providing long-lasting power for medically implanted devices.
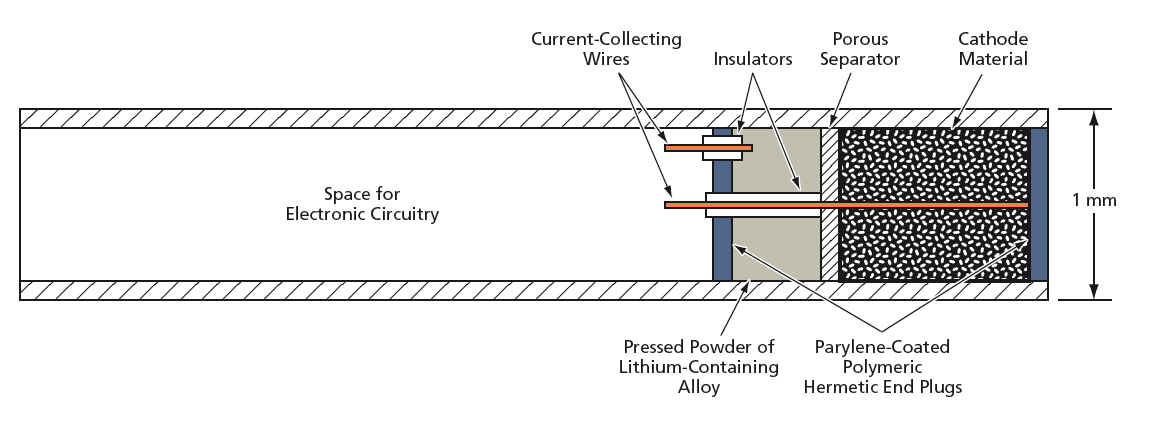
The proposed solution involves utilizing the housing of the implantable device, specifically a tube designed to be placed against the eardrum, as the main battery package. This innovative approach aims to maximize energy density and minimize excess packaging materials. The battery design incorporates a small volume of lithium-based primary battery cathode material, which is compacted and inserted into the end of the implantable tube. A thin porous separator is placed above the cathode, followed by a pressed powder of lithium-containing alloy, creating a compact battery structure.
To ensure safety and functionality, wire current collectors are designed to pass coaxially through the battery while being insulated to prevent short circuits. The battery is sealed hermetically with a waterproof polymer at both ends, followed by a parylene deposition to block potential leaks. The design also allows for the possibility of a hemispherical configuration, where the cathode and anode occupy opposite halves of the tube, separated by the porous separator.
The document emphasizes the novelty of this battery design, which is intended to provide a long-life, low-rate primary energy source that is extremely small (less than 1 mm³). This is particularly crucial for supporting the electronics of implantable medical devices like hearing aids, which require reliable power sources for extended periods.
The Technical Support Package also notes that the information provided is part of NASA's Commercial Technology Program, aimed at making aerospace-related developments available for broader technological, scientific, or commercial applications. For further inquiries or assistance, contact information for JPL's Innovative Technology Assets Management is provided.
Overall, this document outlines a significant advancement in battery technology for medical applications, highlighting the integration of energy solutions within the design of implantable devices to enhance their functionality and longevity.

