According to a proposal, laser-induced acoustic shock waves would be used to lyse cells as needed for biomolecular investigations, including, for example, diagnosis of diseases, pregnancy tests, analyses of genetic molecular structures, and general analyses of cell chemistry. Heretofore, it has been common practice to suspend cells in liquid buffers and to introduce lysing chemicals into the buffers. While chemical lysis is effective, it contributes to the cost and complexity of analysis and creates a problem of disposal of additional chemical waste, especially in situations in which many samples must be analyzed. By eliminating the need for lysing chemicals, the proposed technique would reduce the cost, complexity, and need for post-analysis waste disposal.
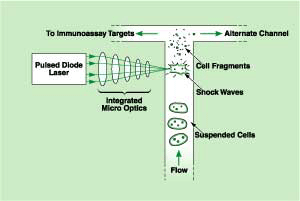
In the proposed technique, a sample of cells would be suspended in a liquid buffer as before, but instead of treating the suspension with lysing chemicals, the suspension would be pumped through an optically accessible channel. The beam from a pulsed diode laser would be focused into the channel (see figure). The energy deposited locally in the buffer by the focused pulses would be sufficient to induce acoustic shock waves, which would lyse cells in or near the focal spot.
This work was done by Robert Stirbl and Philip Moynihan of Caltech for NASA's Jet Propulsion Laboratory. For further information, access the Technical Support Package (TSP) free on-line at www.techbriefs.com/tsp under the Bio-Medical category.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to
Intellectual Assets Office
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109
(818) 354-2240
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Refer to NPO-30410, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Laser-Induced Shock Waves Would Lyse Cells for Analysis
(reference NPO-30410) is currently available for download from the TSP library.
Don't have an account?
Overview
The document outlines a novel technique developed by Robert Stirbl and Philip Moynihan at NASA's Jet Propulsion Laboratory for lysing cells in molecular biological diagnostics using laser-induced shock waves. This innovation addresses significant challenges associated with traditional methods that rely on chemical lysing agents, which complicate the analysis process, increase costs, and create waste disposal issues.
The motivation for this development stems from the operational requirements of field analysis systems that often deal with hazardous bio-agents or non-toxic samples needing rapid processing. Traditional methods necessitate carrying additional lysing reagents, which complicates the system's design and increases its size and expense. The proposed solution leverages the capabilities of coherent light sources, specifically pulsed diode lasers, to generate focused energy packets that create shock waves in the liquid sample. These shock waves can lyse cells without the need for chemical agents, thus simplifying the process and enhancing system portability.
The document details the technical aspects of the method, explaining how the focused laser beam produces a spatially compact deposition of energy near the cells, resulting in their lysis. This approach eliminates the need for chemical reagents, reducing complexity and costs while also addressing post-analysis waste disposal concerns. The technique is particularly advantageous for applications in immunoassays, where accessing cell information is crucial for accurate detection and diagnosis of antibodies.
Additionally, the document emphasizes the novelty of this method compared to existing techniques, which typically involve the use of chemical reagents or enzymes to break down cell membranes for antibody detection. By using laser-induced shock waves, the new method not only streamlines the process but also enhances the efficiency of bioassay systems.
In summary, this document presents a significant advancement in the field of molecular diagnostics, showcasing a laser-based approach that simplifies cell lysis, reduces costs, and minimizes environmental impact. The innovation holds promise for improving the efficiency and effectiveness of various diagnostic applications, particularly in scenarios requiring rapid and safe analysis of biological samples.

