Developed by a team led by Lawrence Berkeley National Laboratory, a self-assembling nanosheet could significantly extend the shelf life of consumer products. And because the new material is recyclable, it could also enable a sustainable manufacturing approach that keeps single-use packaging and electronics out of landfills.
The team is the first to successfully develop a multipurpose, high-performance barrier material from self-assembling nanosheets. The breakthrough was reported online in the journal Nature.
The new nanosheet material overcomes the problem of stacking defects by skipping the serial stacked sheet approach altogether. Instead, the team mixed blends of materials that are known to self-assemble into small particles with alternating layers of the component materials, suspended in a solvent. To design the system, the researchers used complex blends of nanoparticles, small molecules, and block copolymer-based supramolecules, all of which are commercially available.
The new study builds on earlier work. The researchers predicted that the complex blend used for the current study would have two ideal properties: In addition to having high entropy to drive the self-assembly of a stack of hundreds of nanosheets formed simultaneously, they also expected that the new nanosheet system would be minimally affected by different surface chemistries.
This, they reasoned, would allow the same blend to form a protective barrier on a variety of surfaces, such as the glass screen of an electronic device, or a polyester mask.
During experiments at Argonne National Laboratory’s Advanced Photon Source, the researchers mapped out how each component comes together, and quantified their mobilities and the manner in which each component moves around to grow a functional material.
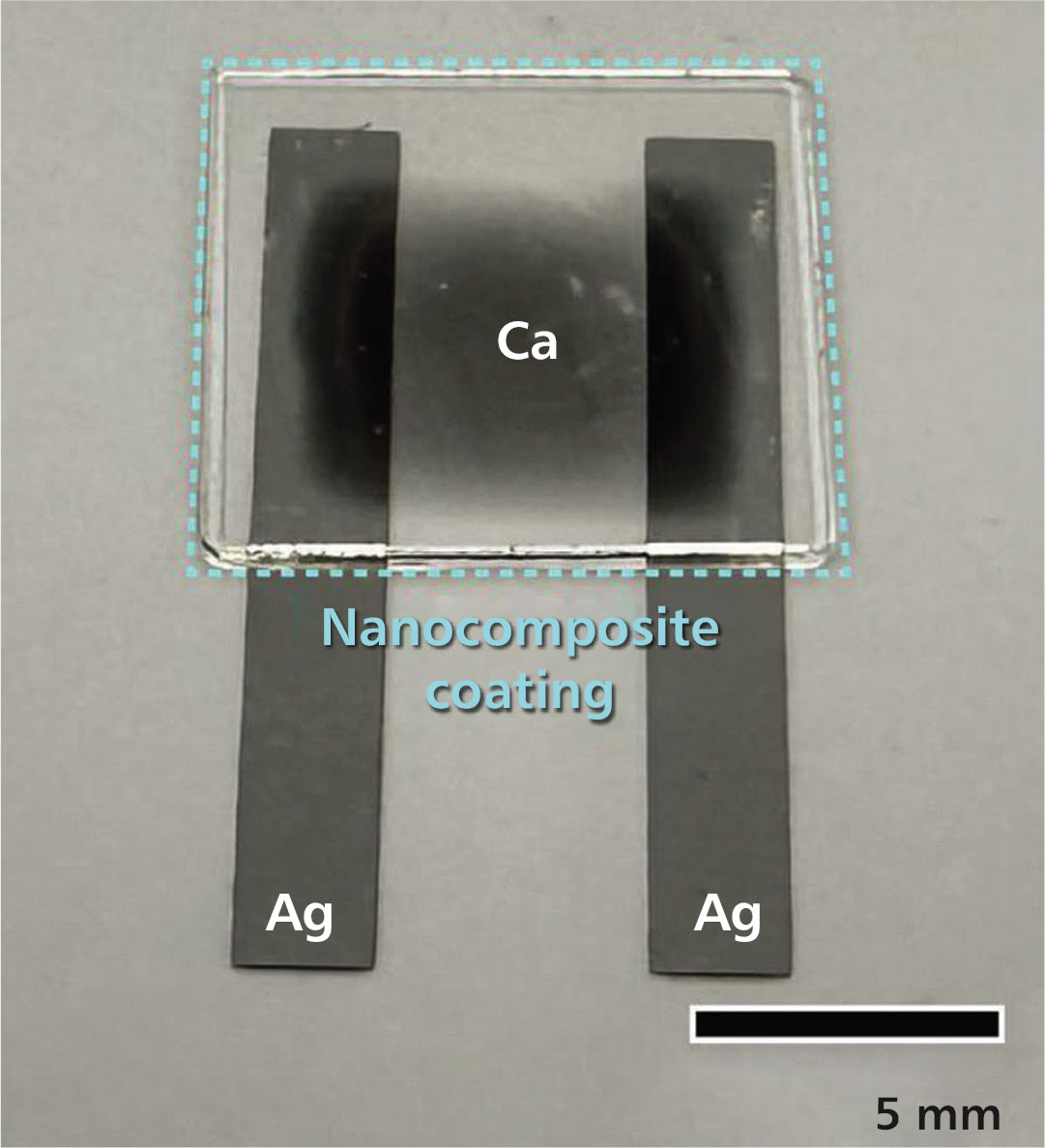
Based on these quantitative studies, the researchers fabricated barrier coatings by applying a dilute solution of polymers, organic small molecules, and nanoparticles to various substrates — a Teflon beaker and membrane, polyester film, thick and thin silicon films, glass, and even a prototype of a microelectronic device — and then controlling the rate of film formation.
Transmission electron microscope experiments at Berkeley Lab’s Molecular Foundry show that by the time the solvent had evaporated, a highly ordered layered structure of more than 200 stacked nanosheets with very low defect density had self-assembled on the substrates. The researchers also succeeded in making each nanosheet 100 nanometers thick with few holes and gaps, which makes the material particularly effective at preventing the passage of water vapor, volatile organic compounds, and electrons, Vargo said.
Other experiments at the Molecular Foundry showed that the material has great potential as a dielectric, an insulating “electron barrier” material commonly used in capacitors for energy storage and computing applications.
The team demonstrated that when the material is used to coat porous Teflon membranes (a common material used to make protective face masks), it is highly effective in filtering out volatile organic compounds that can compromise indoor air quality.
And in a final experiment, the researchers showed that the material can be redissolved and recast to produce a fresh barrier coating.
For more information, contact Theresa Duque at

