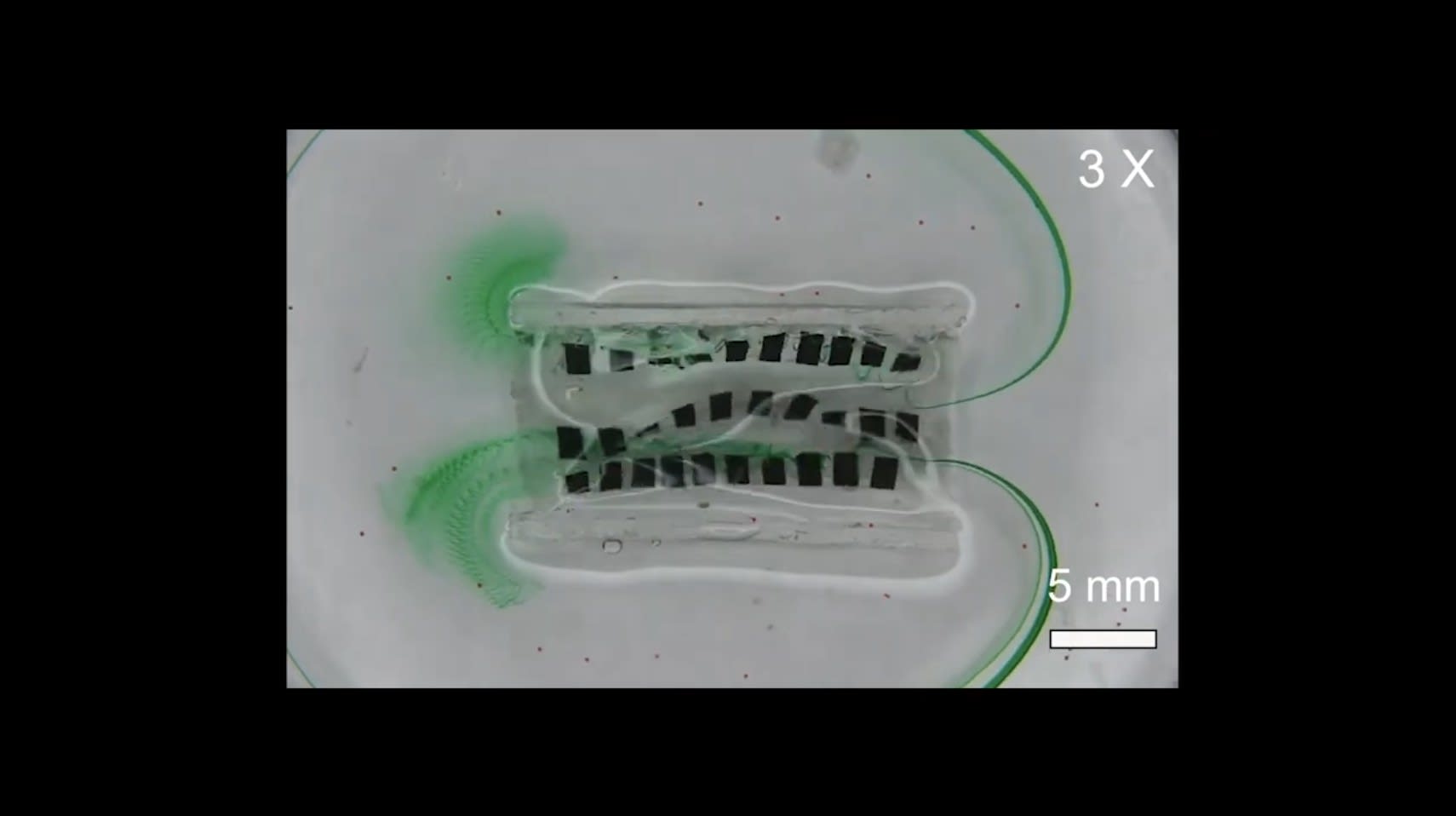
A team of Vanderbilt researchers has developed a wirelessly activated device that mimics the wavelike muscular function in the esophagus and small intestine responsible for transporting food and viscous fluids for digestion.
The soft-robotic prototype, which is driven by strong magnets controlled by a wearable external actuator, can aid patients suffering from blockages caused by tumors or those requiring stents. For example, traditional esophageal stents are metal tubes used in patients with esophageal cancer, mostly in an aging population. These patients risk food being blocked from entering the stomach, potentially causing a dangerous situation where food instead enters the lung.
Restoring the natural motion of peristalsis, the wavelike muscular transport function that takes place inside tubular human organs, “paves the way for next-generation robotic medical devices to improve the quality of life especially for the aging population,” researchers wrote in a new paper in the journal Advanced Functional Materials.
The study was led by Xiaoguang Dong, Assistant Professor of Mechanical Engineering. This work was done in collaboration with Vanderbilt University Medical Center colleague, Dr. Rishi Naik, Assistant Professor of Medicine in the Division of Gastroenterology, Hepatology and Nutrition.
The device itself consists of a soft sheet of small magnets arrayed in parallel rows that are activated in a precise undulating motion that produces the torque required to pump various solid and liquid cargoes.
Here is an exclusive Tech Briefs interview, edited for length and clarity, with Dong and Naik.
Tech Briefs: What was the biggest technical challenge you faced while developing this robotic device?
Naik: It’s twofold. One is implementation — trying to get all of these soft robots in place but also be in a biological plausibility mechanism is very challenging. Being ex vivo is not so difficult but trying to be able to create something that would be able to be placed endoscopically or in vivo in a biological system, that was probably the trickiest part. So, the way we both worked together was to use one of my existing esophageal stents that we have in market. But trying to merge both of our ideas together to be one area was probably the most difficult part overall.
Number two is the size or our magnets; it was just a challenge to keep things as wearable devices. Our novelty of this was peristalsis and undulation but being able to now put these large super magnets into a wearable device, I think will continue to be a challenge we'll face and we’re looking forward to overcome.
Tech Briefs: Can you explain in simple terms how it works?
Naik: We suffer with current modalities to manage peristalsis, especially in areas where there's a peristalsis, or tumor blockade, or issues, but just movement. So, our current model is on the esophagus. This actually would expand to other biological systems in the lung and other areas, too. What we've created was basically a soft robot that has ability to not only implant onto an esophageal stent but also to allow peristalsis and undulation. Why it's novel is that our current paradigms of stenting are basically one-way opening. So, we deploy the device, we are able to obliterate the malignancy, and we can allow people to swallow or have peristalsis.

What is not at all available is the ability to protect ourselves from reflux. A lot of these patients are having difficulties with eating due to lack of peristalsis overall. Trying to recreate that biological valve that we obliterated with our current stent is where this device really can take over.
Dong: The device is a commercial stent. We can integrate on it with some magnetic material, and you can apply external matter to perform an undulation motion. They can generate like a parasitotic-like motion. A kind of traveling wave, we can also generate that. In general, it is wireless; essentially, it's minimally invasive.
Tech Briefs: What inspired your work?
Dong: At the beginning we were looking for applications. We are very good at making soft materials and remotely actuating them. So, when I arrived, I wanted to build something. I was looking around trying to see if there was any application we can target to pump either a solid or viscous fluid. I had been previously working on seed structures you can pump mucus. Eventually, when I talked with Naik, we thought, ‘OK, maybe we can also pump solid and liquid together.’
We tried to integrate it together. I invited him several times to our lab, and we checked our prototype; then he gave us feedback and gradually we morphed into a stent. My student worked on it, and eventually we integrated the design. It's currently ongoing, but that's how it started.
Tech Briefs: You mentioned other biological processes it could be applied to. Can you provide some examples?
Naik: I think the novelty and the broadness of this device is that it's really not limited to just one esophageal stent. So, any area where you have peristalsis and potential strictures from blockages, everything that GI tract really could benefit from the ability to recreate these areas. Then, as we've learned more about reconstruction surgeries, we've also learned that we've damaged all these areas with reconstruction. So, you can go to any area — distal, small bowel, stomach, colon — and recreate peristalsis. It doesn't have to be just tumor. These could be just problems with delayed motility, which are inborn pediatric systems overall. And, certainly, the esophagus and lung system are very similar. So, as we have peristalsis to the esophagus, what the lab has done great on is actually creating even smaller versions for implementation to the lung systems where CELIA clearance, mucus clearance are just so pivotal to protect patients with ongoing inflammation and also to manage patients with blockages or cancers too.
Tech Briefs: What are your next steps? Do you have any plans for further research work, etc.?
Dong: We are currently applying for funding, and we already got some feedback. In the near future, our goal is to fully refine the device — applying some coating so it can stay inside the body for a relatively long time.
That is the first thing, the second thing is we are refining our external attrition system. Currently, on the engineering side we are working on how to make it a practical application.
And then the third point, I want to collaborate with Naik to move on further. If we get external funding, we want to move on to animal testing; we want to test first on small animals like a rabbit and then we want to go to large animals. So, eventually to test the bowel compatibility, the functions inside the body, and a lot of other aspects.
Transcript
No transcript is available for this video.

