A special image-data-processing technique has been developed for use in experiments that involve observation, via optical microscopes equipped with electronic cameras, of moving boundaries between the colloidal-solid and colloidal-liquid phases of colloidal suspensions of monodisperse hard spheres. Such suspensions are used as physical models of thermodynamic phase transitions and of precursors to photonic-band-gap materials. During an experiment, it is necessary to adjust the position of a microscope to keep the phase boundary within view. A boundary typically moves at a speed of the order of microns per hour. Because an experiment can last days or even weeks, it is impractical to require human intervention to keep the phase boundary in view. The present imagedata- processing technique yields results within a computation time short enough to enable generation of automated- microscope-positioning commands to track the moving phase boundary.
The experiments that prompted the development of the present technique include a colloidal equivalent of directional solidification. The interactions between the spheres in these suspensions closely approximate an ideal hardsphere potential, so that the phase behavior becomes, to a close approximation, solely a function of volume fraction (ϕ) of spheres. When ϕ of a given suspension sample is less than a threshold value (ϕf = 0.494) denoted the freezing volume fraction, the suspension is in the colloidal-liquid phase, in which the spheres are disordered and free to diffuse throughout the entire volume of the sample. When ϕ exceeds another threshold value (ϕm = 0.545) denoted the melting volume fraction, the suspension is in the colloidal-solid phase, in which the sample is crystalline in the sense that each sphere is “caged” by its neighbors and thus restricted to small movement about a lattice point. Between ϕf and ϕm is a regime of coexisting colloidal liquid and colloidal solid.
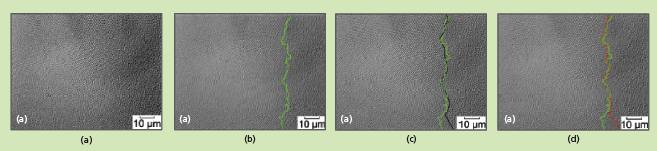
At the beginning of an experiment, a suspension is prepared at ϕ well below ϕf and placed in a cell. Then through slow evaporation or gravitational sedimentation, the spheres become concentrated toward one end of the cell, where crystallization starts when ϕf is reached. When the sphere size falls within a range accessible to optical microscopy, the disordered (liquid) phase and the ordered (solid) phase (and, hence, the boundary between them) are visible to, and clearly distinguishable by, a human observer. However, prior image-data-processing techniques do not enable automated distinction between regions of order and disorder in images of closely packed spheres.
In the present technique, automated distinction (see figure) is made possible by differences between the motions of the spheres in the liquid and solid regions. In particular, the technique exploits the fact that in the solid phase, the spheres are restricted to their small “cages,” whereas in the liquid phase, the spheres are free to move. Consequently, when images are averaged over successive frame periods, the liquid region tends to become blurred or gray while the solid region retains a higher degree of contrast, showing the spheres as individual particles. Each frame-averaged image is subjected to a brightness-slicing, a cleaning (noisesuppression), and a particle-finding operation. These operations utilize the brightness and contrast differences between the solid and liquid regions. Then the image region showing particles is deemed to be the solid region and the phase boundary is located accordingly.
This work was done by Mark McDowell and Richard B. Rogers of Glenn Research Center and Elizabeth Gray of Scientific Consulting, Inc. For more information, download the Technical Support Package (free white paper) at www.techbriefs.com/tsp under the Physical Sciences category.
Inquiries concerning rights for the commercial use of this invention should be addressed to NASA Glenn Research Center, Innovative Partnerships Office, Attn: Steve Fedor, Mail Stop 4–8, 21000 Brookpark Road, Cleveland, Ohio 44135. Refer to LEW-18157-1.

