Epoxies are versatile polymer systems that are “go-to materials” for electrical, electronic, and microelectronic systems, especially in applications where outstanding electrical insulation properties are needed. Their wide usage is due to their excellent adhesion to a wide variety of substrates, superb chemical and heat resistance, and long-term durability. They are serviceable for bonding, sealing, coating, and encapsulating/potting applications.
The primary focus of this article is twofold; the first is to discuss the electrical insulation properties as they pertain to epoxies. The other is to delve into the variation of these properties, based on the chemistry of the system (especially the role of the curing agent) as well as the operating conditions of the application.
Prior to curing, an epoxy consists of a resin and curing agent, which when mixed, polymerize and form a cured matrix. There are many different types of epoxy resins and curing agents. When combined, they create distinct cross-linking patterns resulting in different attributes of the polymerized system. The choice of the curing agent depends not only on the electrical insulation values desired, but also on other parameters such as operational temperatures, chemical resistance, and physical strength requirements, among others. Another consideration in selecting the hardener is to assess its processing capabilities and constraints. We will start by discussing some of the fundamental electrical insulation properties, i.e. dielectric constant, dissipation factor, dielectric strength, and volume resistivity. We will then correlate these values in terms of processing to the ultimate properties obtained with various groups of curing agents, including aliphatic amines, polyamides, cycloaliphatic amines, aromatic amines, anhydrides, lewis acids, and imidazoles.
Dielectric Constant
Also known as relative permittivity, the dielectric constant indicates the ability of a material to store electrical energy in response to an electric field. It is a dimensionless number defined as the ratio of the permittivity of a material relative to that of a vacuum, where permittivity is a measure of the electrical energy stored as a result of an applied voltage. Generally, a low value (2-5) is desirable for epoxies and other materials intended for use as electrical insulators, although in certain applications, a mid-level dielectric constant (6-12) is required.
The standard test method for measuring the dielectric constant of a solid electrical insulating material is ASTM D150. It involves placing a sample of the material between two capacitor plates and measuring the resulting capacitance — the ability to store an electrical charge. This is then compared to the capacitance of the same plates with air or a vacuum between them. The resulting ratio is the dielectric constant of the material.
For a cured epoxy system, the dielectric constant varies with temperature, frequency, and filler. For instance, a particular system may have a dielectric constant that increases with temperature (3.46 at 23 °C, 3.55 at 100 °C, and 4.24 at 150 °C) for a 60- Hz application, but fluctuates with temperature (3.28 at 23 °C, 2.99 at 100 °C, and 3.87 at 150 °C) for a 1-KHz application. In general, but not always, the dielectric constant increases with higher temperatures and decreases with higher frequencies. Essentially, epoxies lose some of their insulation capabilities at higher temperatures, but exhibit better insulation properties for higher frequencies. The addition of mineral filler particles increases the dielectric constant of a particular epoxy system slightly, while metallic fillers will have a more notable impact.
Dissipation Factor
The dissipation factor (DF) is a measure of power loss in a material subjected to an alternating electric field. According to the standard ASTM D150, the DF is the ratio of the power dissipated to the power applied. (An additional standard, ASTM D2520, is recommended for characterizing DF at microwave frequencies.) A lower DF is desirable in order to reduce the heating of the material and minimize the impact on the surrounding circuit. Dissipation factor can be a very useful measure of other characteristics of a material, such as degree of cure, voids, moisture content, and contamination. Over time, a significant change in DF can occur when the operational conditions are too severe for the cured system.
The DF is typically 0.003 to 0.030 at 1 KHz, and up to 0.050 at 1 MHz. At ambient temperatures, DF (in most cases) increases as the frequency gets higher. As temperature rises, the effect on DF varies greatly depending on the operating frequency and the specific chemistry. For example, at 1 KHz, the dissipation factor of a particular system falls from roughly 0.02 to less than 0.01 as the temperature increases from ambient to 125 °C, at which point the DF rises dramatically, nearly reaching 0.8. For the same system operating at 8.5 × 109 Hz, the DF rises gently from 0.02 and then levels off below 0.05 as temperature increases.
The overall effect of mineral fillers is to somewhat increase the DF, although the degree of change is highly dependent on temperature and frequency. For metallic fillers, the DF increases greatly.
Dielectric Strength
Another significant criterion in assessing isolation properties of an epoxy is the dielectric strength, which is often expressed in volts/mil (1 mil = 0.001 inch). This is defined as the maximum voltage that can be applied across a sample of the material without causing dielectric breakdown. The resistance of the material in dielectric breakdown decreases rapidly and it becomes electrically conductive.
ASTM D149 is the standard test used to determine theoretical dielectric strength. The test method consists of placing a sample of the material between two electrodes in water or oil and applying a voltage across the electrodes. The voltage is then increased at a uniform rate from zero until the point at which the material exhibits burn-through punctures or begins to decompose. The resulting breakdown voltage is divided by the sample thickness to derive the intrinsic dielectric strength. Higher values indicate better electrical insulation characteristics.
In practice, dielectric strength is highly dependent on the thickness of the material, with thinner samples having higher values per unit thickness. For example, the dielectric strength values for epoxy systems could be as high as 2,000 volts/mil for a 0.010" sample, gradually reducing to about 425-475 volts/mil for a 0.125" specimen. Thicker sections tend to retain this dielectric strength value of about 425-475 volts/mil at ambient temperatures. Thus, one of the major factors in assessing an epoxy’s dielectric strength is a very precise elucidation of the test method used as it relates to the thickness of the cured epoxy. Dielectric strength generally decreases as operating temperature or frequency increases. Since dielectric strength is application dependent, it is important to validate epoxies for their dielectric strength for specific uses, especially for those involving high currents.
Most non-conductive mineral fillers have little effect on the epoxy’s dielectric strength, and metallic fillers decrease the dielectric strength depending on the nature of the filler and filler loading.
Surface and Volume Resistivity
Resistivity is the ability of a material to resist the passage of an electric current under specified conditions of applied voltage, temperature, and time. Surface resistivity, expressed in ohms, characterizes the resistance to leakage current along the surface of a material, while volume resistivity, expressed in ohm-cm, measures the resistance to leakage current through the body of a material. ASTM D257 is a widely used standard for measuring volume resistivity in insulating materials.
For unfilled epoxies, volume resistivity typically exceeds 1012 ohm-cm at 25 °C. Most mineral fillers have a marginal effect on volume resistivity, while certain metallic fillers will lower the volume resistivity. Epoxies with significant loadings of some metallic fillers, such as silver, are excellent electrical conductors. Other metallic fillers, such as stainless steel, will lower the volume resistivity, but not transform the epoxy into a conductor.
Adding temperature has an interesting effect on epoxies. When the epoxy is an insulator, an increase in heat causes a decrease in the volume resistivity. However, adding heat to an electrically conductive epoxy increases the volume resistivity, i.e. causes a lowering of the electrical conductivity.
Selecting a Curing Agent and its Effect on Electrical Properties
As noted earlier, the specific composition of resin and curing agent has a profound impact on the properties of a cured epoxy system. We will reference the resin as “standard-type” bisphenol A. There are three major types of curing agents for epoxies: amines (the most common), anhydrides, and catalyzed systems (Lewis acids, typically boron trifluorides, and imidizoles among others). Each group has, in parts, distinct electrical insulation characteristics. These are considered in conjunction with their processing and handling parameters.
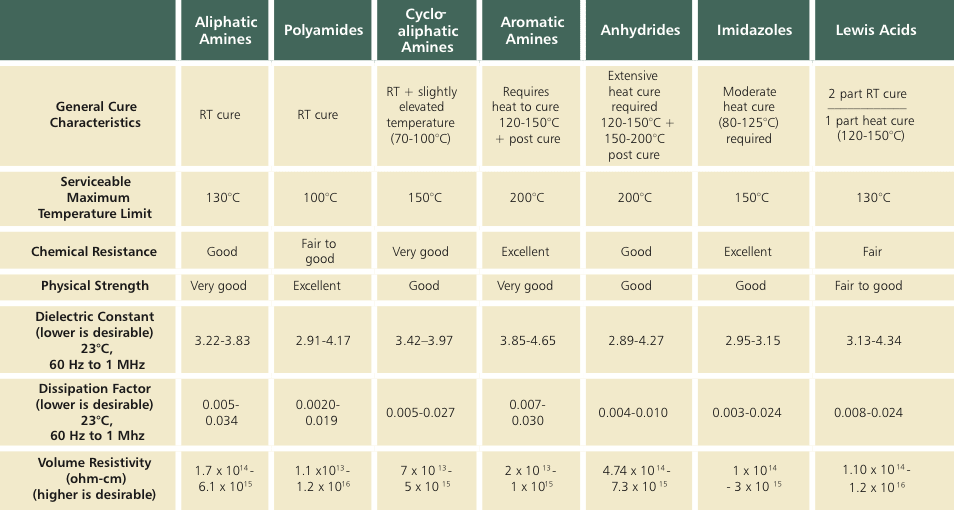
Historically and functionally, one of the most prominent classes is the aliphatic amines. They are lower in viscosity, cure readily at room temperature, and some are serviceable for continuous operating temperatures up to 130 °C. They are widely used in various bonding, sealing, and potting applications, and have outstanding electrical insulation properties. Additionally, their chemical resistance and physical strength properties are quite good. The mix ratio for these aliphatic amines tends to be uneven (for example: 100:12), and not quite as forgiving as other amine systems. Although they cure nicely in thin sections, they are generally exothermic and are not typically cured beyond ¼" in thickness.
A second category of amines is higher molecular weight amine adducts (amido amines) — the most common being polyamide curing agent. These curing agents cure readily at room temperature but tend to be higher in viscosity. Their mix ratios are very forgiving and user friendly (a 1:1 mix ratio is rather common for this class). They are among the best room temperature curing systems in terms of their electrical insulation properties. However, the temperature resistance conferred upon the system is not as high as their aliphatic counterparts. They are usually serviceable from ambient to around 100 °C on a continuous basis. Polyamides have very low dielectric constants along with other outstanding electrical insulation values at ambient temperatures. They are typically not exothermic, and can be cured readily to thicknesses of up to 2". In fact, there is a sub-group in this class with so low an exotherm that it can be cured to thicknesses of 5-6". Another interesting feature is that it confers a modicum of toughness to the cured system.
Another important group of amines is the cycloaliphatic amines. Like other amines, they have very good electrical insulation properties. Featuring a low to moderate viscosity and room temperature curing, heat is typically added to optimize their cured properties. However, the heat required is not particularly high (70-100 °C). They surpass both polyamides and aliphatic amines in terms of their temperature and chemical resistance, with some systems that are serviceable up to 150 °C continuously. Cycloaliphatic amines have more tolerant mix ratios than aliphatic amines, but less so than polyamides. They vary in the exotherm, but tend to be on the lower side. There are many different cycloaliphatic amines that are commercially available with each one presenting a slightly different electrical insulation profile, although all providing relatively excellent insulation values.
Aromatic amines are the mainstay for high-temperature and chemical-resistance applications. They require higher temperature for curing than the other amines discussed thus far. Usually, they require curing at 120-150 °C with post curing at 150-200 °C, and have a low to moderate viscosity at room temperature. While some of the aromatic amines may have relatively slightly lower electrical insulation values than other amines at ambient temperatures, they are still very robust in this regard and are widely used, primarily due to their chemical and temperature resistance attributes. Most are serviceable up to temperatures of about 200 °C on a continuous basis. They have a very low exotherm, a working life of a number of days, and are well suited for larger castings. Their mix ratios are typically more complex than 1:1 or 2:1; however, they are forgiving in nature.
The second major category of curing agents is anhydrides, sometimes called acid anhydrides. Of all the major groups, their main applications are for potting and encapsulation. In fact, they are used primarily because of their unsurpassed electrical insulation properties. Realistically, however, anhydrides require extensive heat for cross-linking with cure schedules of 120-150 °C for 8-12 hours, followed by post curing to optimize on some of those properties. They are low in viscosity and have an extremely low exotherm, with the working life extending in many cases for weeks at room temperature. Most have exquisite temperature resistance and other superior physical strength properties such as tensile strength and modulus, etc.
Catalyzed systems form the third group of curing agents. They are available in one- and two-part systems. Lewis acid systems, primarily boron trifluorides, are effective for applications involving faster curing requirements and superior temperature resistance. This group tends to be exothermic and when used in two-part systems, their mix ratios are a bit more restrictive. When utilized as a one-part system, they are also exothermic and require high temperatures for curing (150 °C). A major application for one-part systems is impregnation, but can be readily formulated for potting/encapsulating type applications.
Imidizoles are usually classified as catalyzed systems although they are not Lewis acids. With very long open times >12 hours at room temperature, they also feature moderate viscosity and outstanding temperature and chemical resistance. They require moderate temperatures to cure (80-120 °C). Mix ratios are usually uneven (for example, 100:5), but are more forgiving than other amines. As with other catalyzed systems, imidizoles tend to confer lower elongation and higher modulus values to the cured entity. They are used primarily for bonding and sealing applications, and can also be employed in conjunction with other curing agents to enhance the temperature resistance profile. A summary of the curing agent groups discussed can be found in the table.
Conclusion
Epoxies are extensively used for bonding, sealing, coating, and potting and encapsulation applications. As this article demonstrates, all epoxy systems are inherently good insulators, especially when evaluated by dielectric strength, volume resistivity, dielectric constant, and the dissipation factor. They are outstanding electrical insulators; however, there are subtle differences in the electrical properties based on the class of curing agent used. Ultimately, the choice of the curing agent depends upon the operational conditions that the epoxy will experience and the processing limitations dictated by the application itself.
This article was contributed by Master Bond, Hackensack, NJ. For more information, Click Here .

