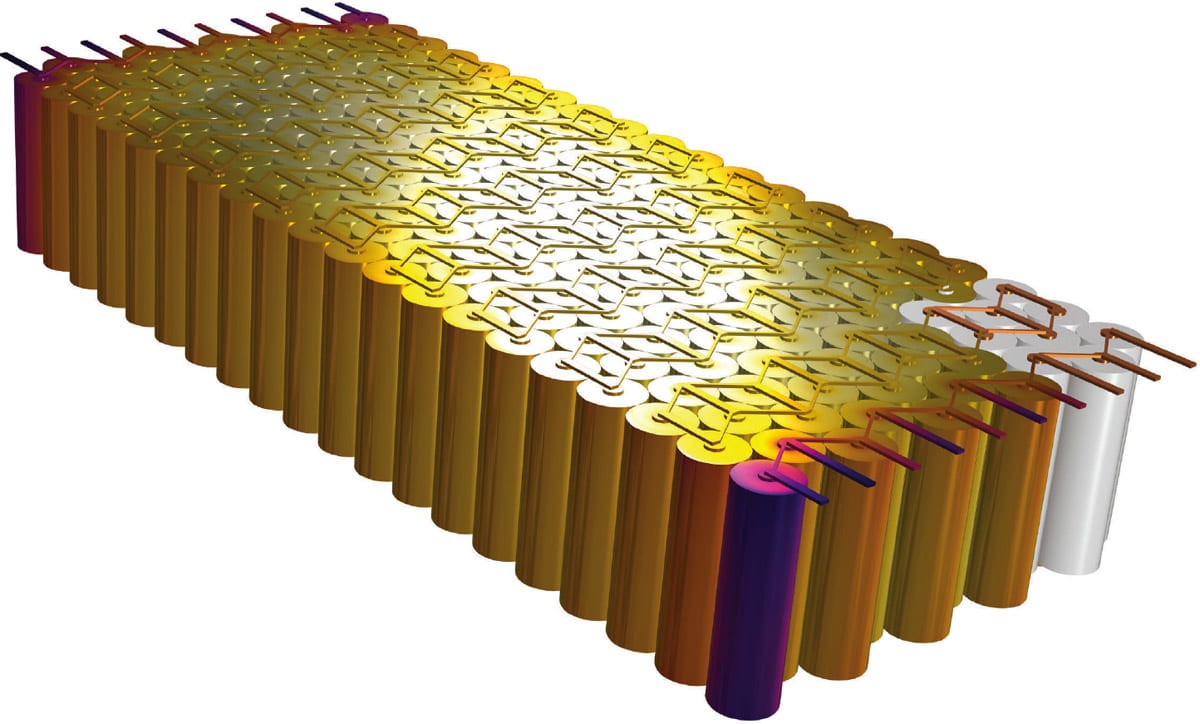
Cylindrical cells are one of the most common battery types used in electric vehicles. Tesla recently improved on the typical cylindrical cell design, developing a new tab design that allows for a much larger cell. [1,2] With this design, a single battery pack only requires 900 cells — as opposed to the roughly 7,000 cells contained in a traditional pack — which offers multiple advantages: It is easier to manufacture the battery pack, and there are fewer cells as well as fewer cans and contacts per pack, which means that there is a larger amount of active battery material in relation to the can material and other nonactive materials.
A larger cell radius would normally increase the energy losses in a cell. However, in this article, we will explore how it is possible to make larger cells without also generating larger energy losses. We will do this by building two sets of models: one tha describes the standard design with traditional tabs and one that describes Tesla’s new design according to its patent. [1,2]
The Cylindrical Cells
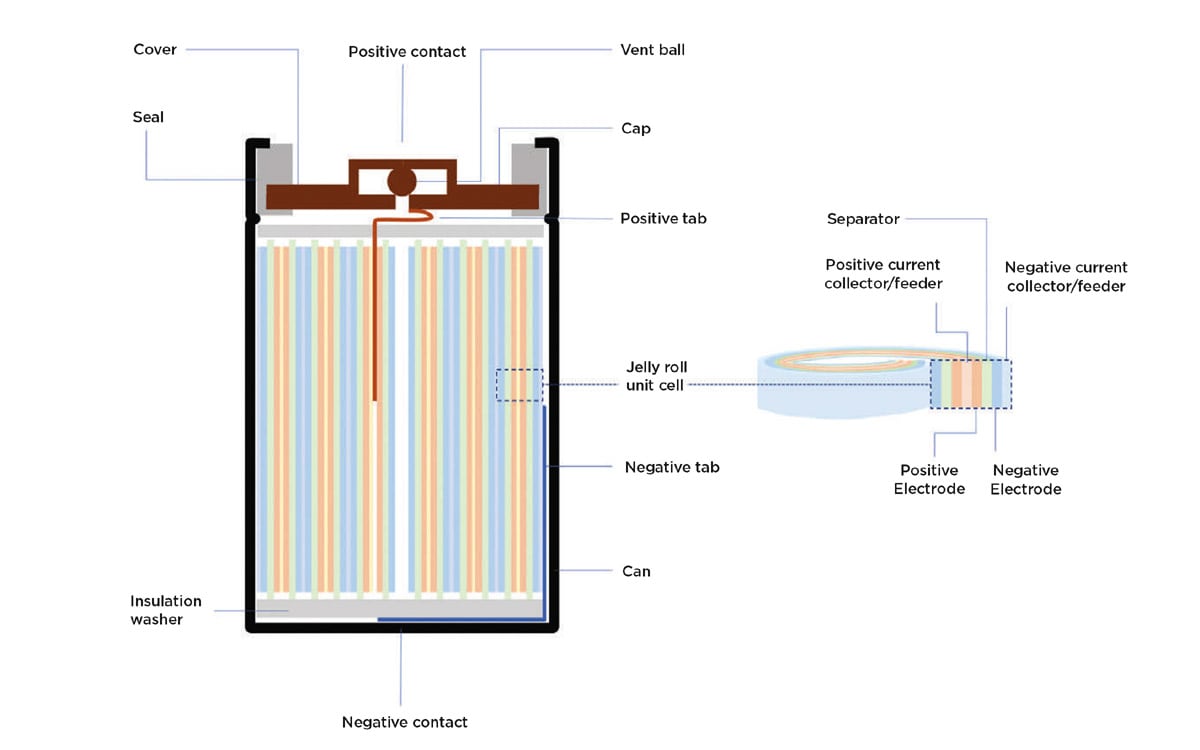
In a traditional design (Figures 1 and 2), each cylindrical cell consists of:
A steel can with the bottom of the can forming the negative contact of the cell
The top cap, which forms the cell’s positive contact
A cover that together with the top cap seals the top of the cell
A so-called “jelly roll,” consisting of layered components including a positive and a negative electrode, a separator, and metal foils, sometimes called current collectors or current feeders, that conduct current into and out of the electrodes
Two end tabs: one positive end tab welded to the innermost section of the positive current collector and one negative end tab welded to the outermost section of the negative current collector.
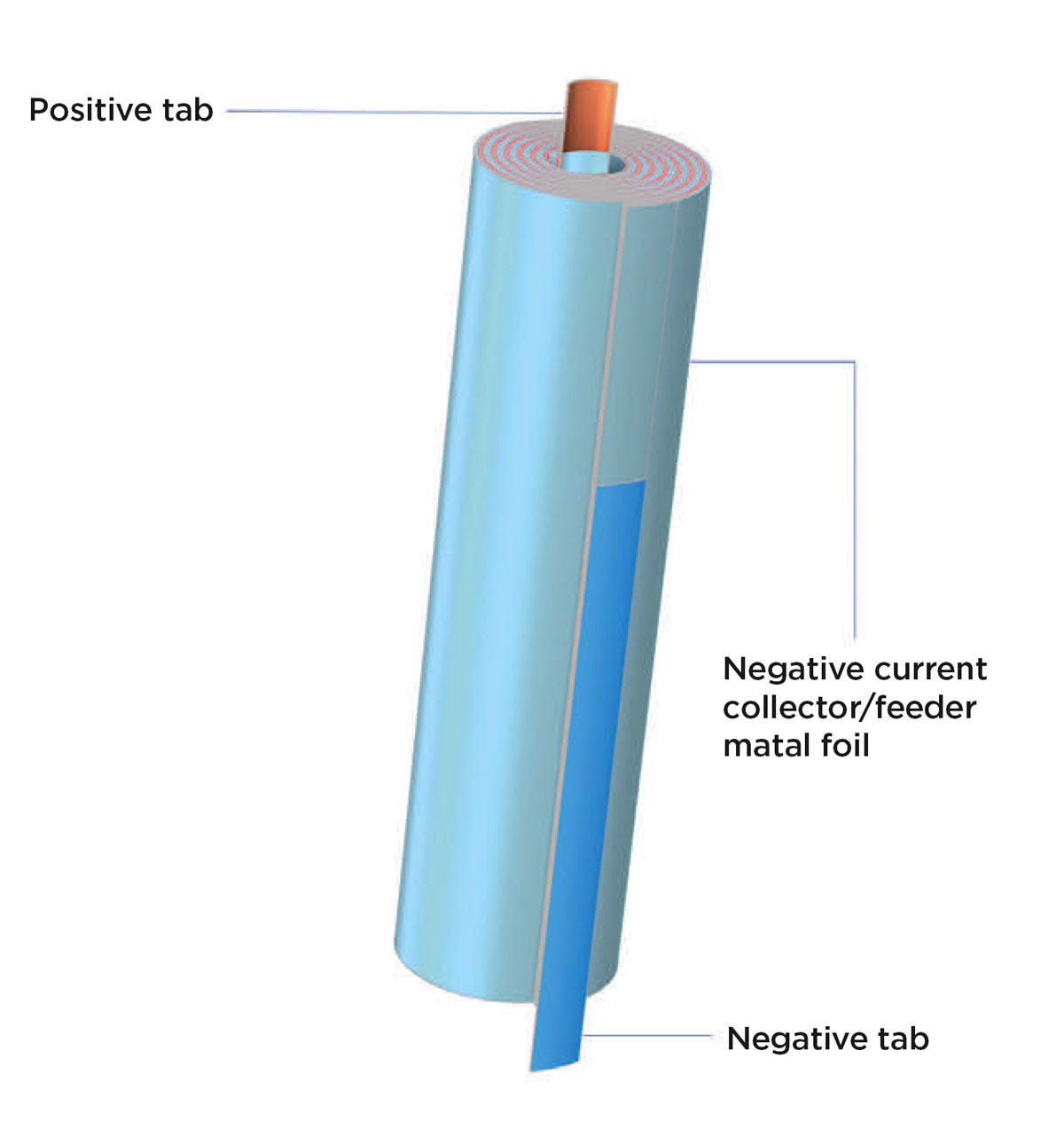
The jelly roll is formed by stacking the electrodes, the separator, and the metal current collector/feeder foils, and then rolling or coiling the stack around the central axis of the cylindrical cell.
When the jelly roll is inserted into the can, the negative tab is folded to make contact with the bottom cover, while the positive tab makes contact with the top cover (Figure 1).
Higher Energy and Power Density in Cylindrical Cells
Increasing the radius of a cylindrical cell increases the volume of active material at a rate that is proportional to the square of the radius, while the amount of steel in the lateral area of the cylindrical can increases linearly with the cell radius. For example, the volume-to-outer-surface ratio of the 18650 cell (18 mm in diameter and 65 mm in height) is 3.95 mm, while the value for the 4680 cell (46 mm in diameter and 80 in height) is 8.93 mm – this is more than twice the volume per unit of outer surface.
The larger radius of a cylindrical cell also means a larger resistance for the current from the electrodes to the tabs. Tesla’s patent describes how this is solved by replacing the traditional design’s thick, welded end tabs with thinner tabs. [1,2] (It is also possible to insert several tabs into a traditional cell, but this involves a more complicated manufacturing process with more welds that may make the jelly roll more susceptible to short circuits and internal hot spots due to the formation of burrs. [3] The tabs in the new design are formed by extending the foils beyond the height of the other jelly roll layers (upward for the positive foil and downward for the negative foil) and cutting slits into the extended parts of the foils (Figure 3).
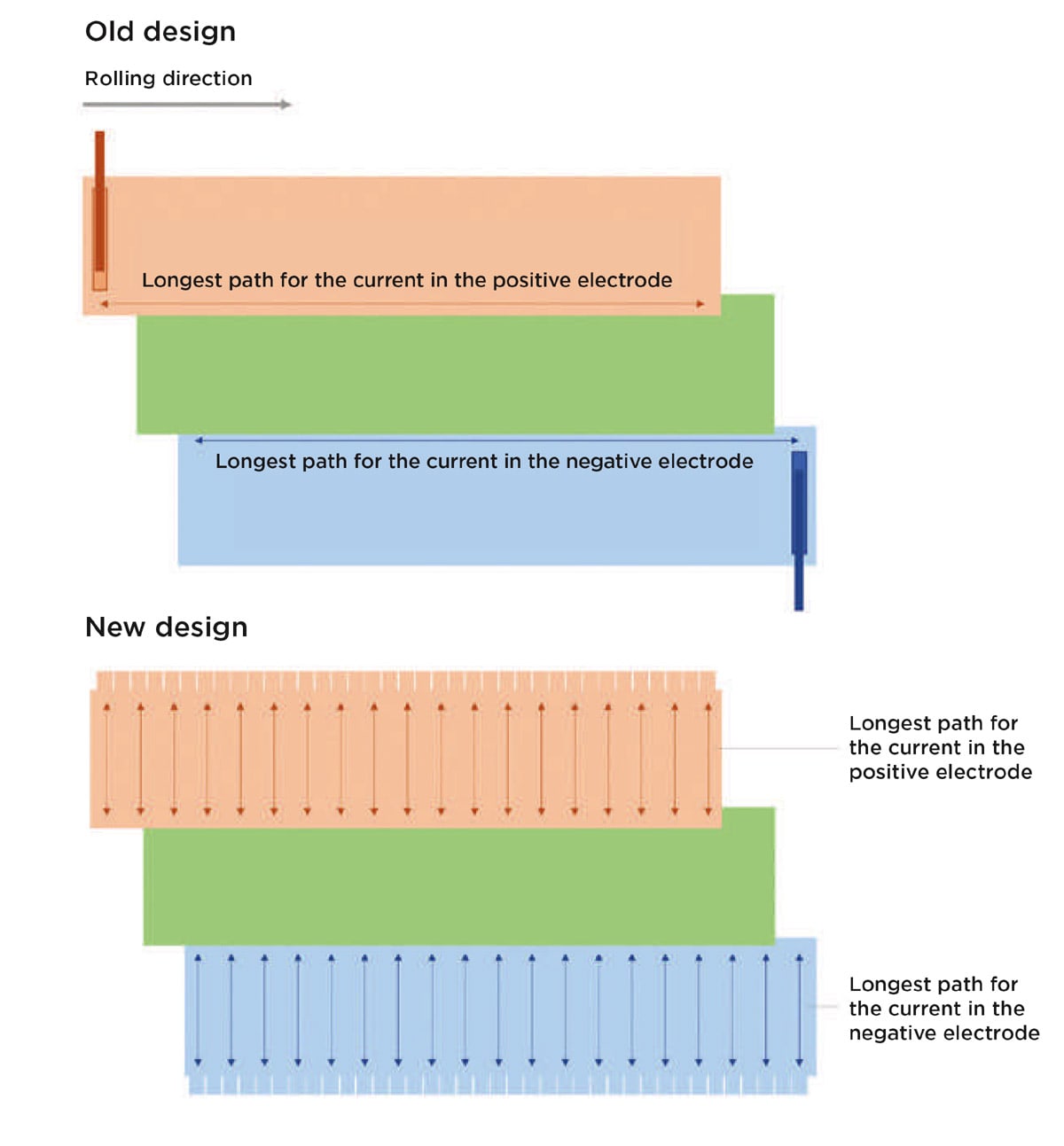
The cuts allow the tabs to be folded without wrinkling the foil when the jelly roll is rolled. [2] The shorter path for the current, as shown in Figure 3, not only makes it possible to design cells with larger radii, but it also allows for cells with greater height, which adds some freedom in the pack design. In addition, the metal foils have excellent thermal conductivity, which enables greater control of the temperature of the cell.
The Models and Results
We can conclude from literature and from our reasoning here that the new tab design should have better performance. The question is how much better.
To investigate the performance of the two designs, we will use a Newman model for the lithium-ion battery, which is the most accurate model for this battery. [4,5] We will select the 18650 type of cell, i.e., the smaller cell, for our models. Even for this smaller cell, the comparison between the old and new design should demonstrate the trend in performance.
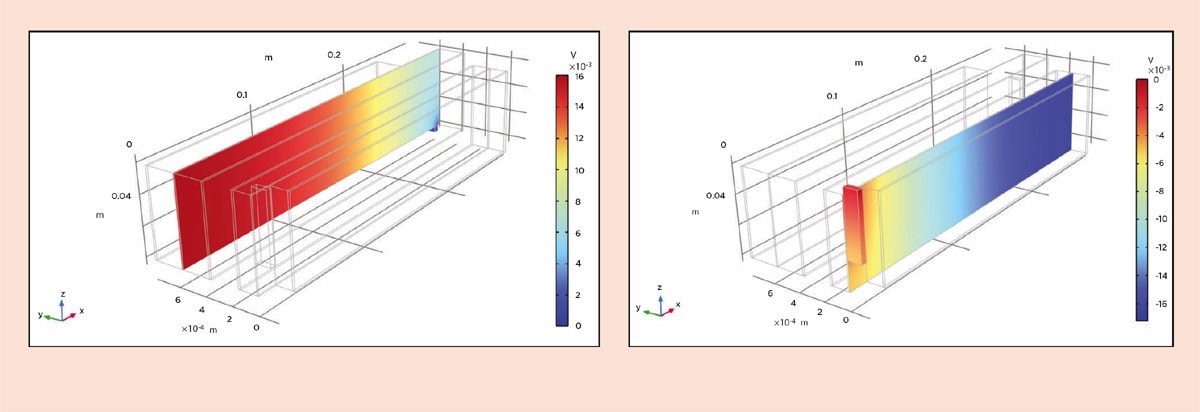
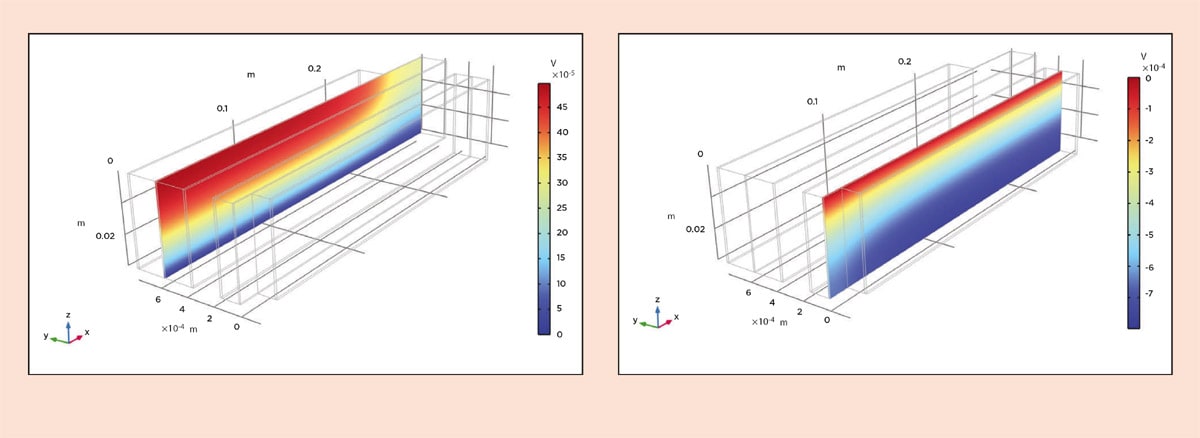
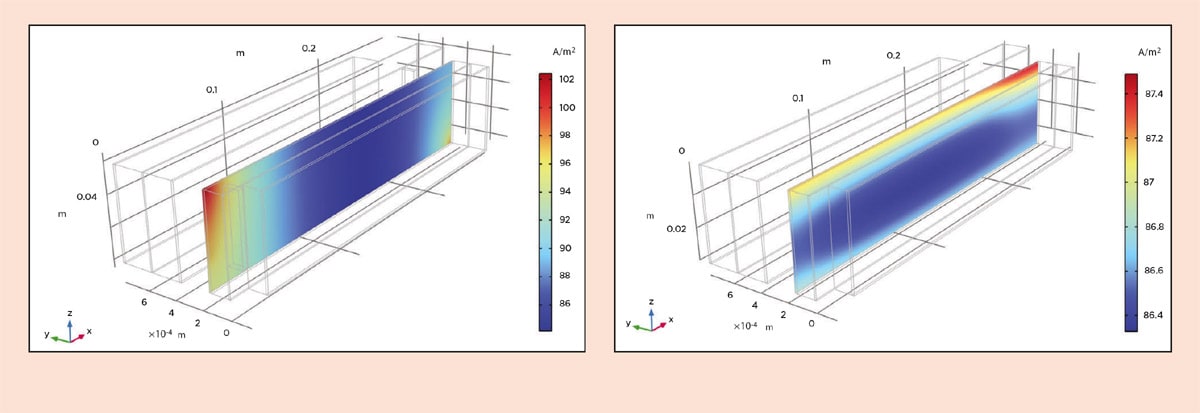
Let us look at the performance during recharge at 1C, meaning that the batteries are charged in one hour at a constant current. We can start by looking at the ohmic losses in the old design in Figure 4. Note that since the jelly roll is difficult to visualize, we have virtually unrolled it. Also note that the y direction is multiplied by a factor of 100 in the visualization. We can see that at the beginning of the recharge, the ohmic losses are 16 mV in the copper foil and 17 mV in the aluminum foil. In the new design, we can see from Figure 5 that the losses in the copper foil are around 0.5 mV and around 0.8 mV in the aluminum foil.
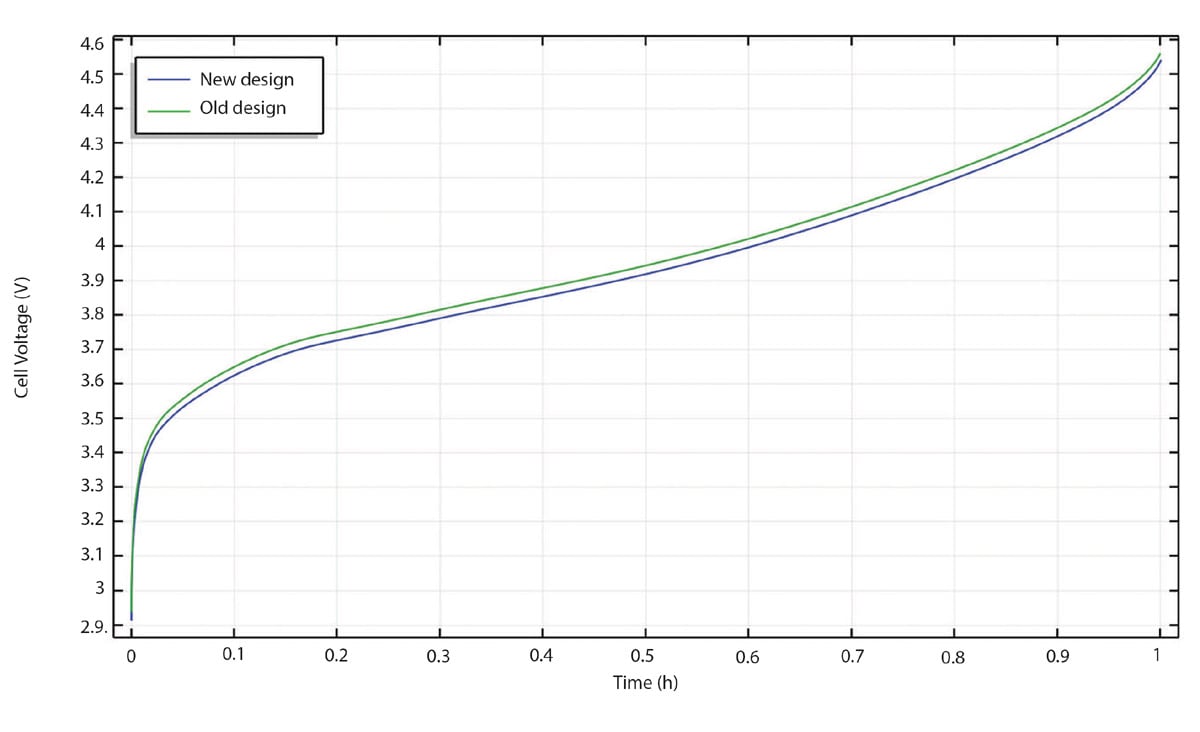
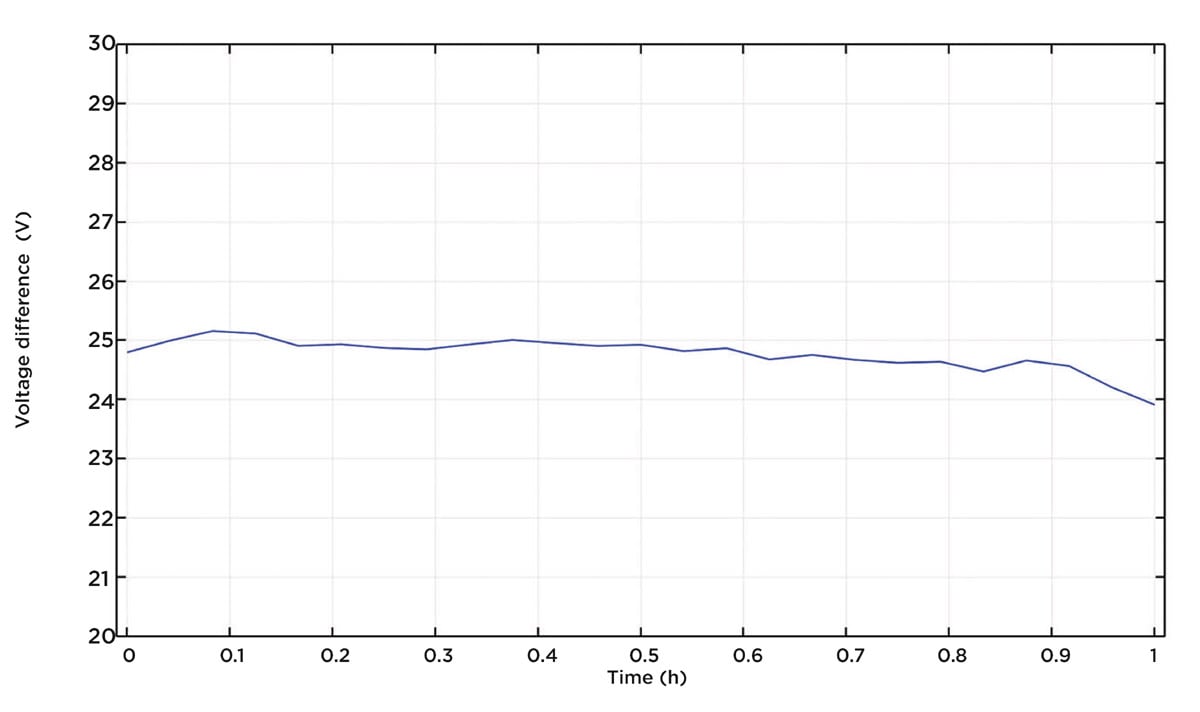
Now, let us look at Figure 6 to compare the recharge curves for the old and new design. We can see that the new design performs better. If we plot the difference in potential between the two curves in Figure 6, we can see that the improvement is almost constant over the whole recharge process, as shown in Figure 7. The new design performs about 24-25 mV better over the whole recharge period, which is a substantial improvement.
We can also analyze the current density distribution in the separator ( Figure 8). In the old design, the current density at the beginning of the recharge is about 22 percent higher at the tabs compared to in the interior of the roll. As a constant load or recharging cycle progresses over time, this distribution becomes more uniform.
However, for a battery subjected to dynamically shifting charges and recharges, such as for a car undergoing repeated acceleration and regenerative braking, the nonuniform current distribution is of importance: It results in nonuniform utilization of the battery, which accelerates aging. With the new design, the current density is much more uniform, with a difference of only 1.3 percent between the largest and smallest value (Figure 8).
Conclusion
The models show that the new design has better performance and generates less heat over the whole recharge-discharge cycle. This difference would be even larger if the cells’ radii were increased. The new design is also less susceptible to aging, due to its more uniform current density distribution.
Tesla has neither sponsored, endorsed, nor entered any other relationship with COMSOL concerning this article.
This article is based solely on published information concerning Tesla’s tab design for its battery pack.
This article was written by Ed Fontes and Henrik Ekström, Chief Technology Officer and Technology Manager of Electrochemistry, respectively, of COMSOL (Burlington, MA). For more information, visit here .
References
- International Application, PCT/US2019/059691, WO 202/09697.
- “New Tabless Battery Patents Get Better Visualizations”, The Tesla Space.
- X. Yao and M. G. Pecht, “Tab Design and Failures in Cylindrical Li-ion Batteries” IEEE Access, vol. 7, pp. 24082– 24095, 2019.
- H. Ekström and E. Fontes, “Battery Modeling” white paper, COMSOL.
- J. Newman and K.E. Thomas-Alyea, Electrochemical Systems, John Wiley & Sons, pp. 517, 2004.

