Battery engineers targeting electric vehicles (EVs) continue to research designs with solid-state electrolyte because of the alluring twin promises of significantly higher energy densities – which lead to longer driving range – and greatly enhanced safety that comes with eliminating liquidous electrolytes. Additional presumed advantages for solid-state batteries are quicker recharging and longer lifespan – not to mention the potential to reduce the amount of critical, high-cost minerals required for lithium-ion battery chemistries.
Solid-state technology is far from perfected for automotive-scale production and with increasing global interest in EVs as an answer to climate change and fossil-fuel supply concerns, barely a May passes without the breathless announcement of some new battery breakthrough. But promising recent research and development on several fronts appears to be bringing solid-state battery designs closer to production readiness – and it was reported in late January, 2022, that Chinese automaker Dongfeng Motor launched a demonstration program of 50 Dongfeng E70 EVs using solid-state batteries developed in collaboration with Ganfeng Lithium. Although scant detail is available, the Ganfeng solid-state batteries employ a “flexible-diaphragm” technology to facilitate lithium-ion travel while also curbing formation of lithium dendrites, a common problem that inhibits energy retention and recharging performance.
Process as a Pathway
Partially addressing the issue of dendrite formation as well as other performance- and longevity-related factors, research announced by a research team from MIT and Brookhaven National Laboratory indicates a comparatively quick and low-cost process may help eliminate a costly manufacturing step that currently is necessary to achieve the best performance from solid-state batteries. The researchers believe their method can bring more efficiency and value to the solid-state battery manufacturing process. The researchers looked at the critical interface between the solid-state electrolyte (usually some type of ceramic material) and the cathode and anode materials on each side of the electrolyte. The electrode materials typically are sintered at high heat to the electrolyte to assure physical bonds that yield the best possible conductivity. But now, to achieve the best material bonding, special coatings were needed at the interface of the electrolyte and the cathode layer. Sintering, which for ceramic materials usually is performed at temperatures of 1,000 °C (l832 °F) or higher, causes atoms from each material to migrate into the other to form bonds.
The team's experiments, said an MIT News article summarizing the work, showed that at temperatures anywhere above a few hundred degrees, detrimental reactions take place that increase the resistance at the interface, but only if carbon dioxide is present, even if in meager amounts. The researchers demonstrated that sintering in the total absence of carbon dioxide and maintaining a pure oxygen atmosphere, could create very good bonding at temperatures up to 700 degrees – and without forming the undesirable, resistance-inducing compounds.
The performance of the cathode-electrolyte interface made using this method, said professor of nuclear and materials science and engineering Bilge Yildiz, who also coauthored the technical paper describing the research in the journal Advanced Energy Materials, was “comparable to the best interface resistances we have seen in the literature,” she told MIT News. But those favorable resistances were achieved using the extra step of applying coatings. “We are finding that you can avoid that additional fabrication step, which is typically expensive,” Yildiz added.
The researchers now are analyzing the durability of these specially sintered bonds during extended battery cycling. But the new findings potentially could rapidly be applied to battery production, Yildiz said. “What we are proposing is a relatively simple process in the fabrication of the cells. It doesn't add much energy penalty to the fabrication” and the added costs should be negligible.
Toyota, which has its own development program for solid-state batteries, also is concerned with the potential for long-term performance degradation related to lithium dendrite formation. Toyota Motor Corp. CTO Masahiko Maeda said in a mid-2021 virtually conducted media briefing attended by SAE Media that in the company's ongoing solid-state battery research, “we found that short service life was an issue. To solve this and other issues, we need to continue development, mainly of solid electrolyte materials.” He also stressed that the company would prefer to first launch solid-state batteries in hybrid-electric vehicles (HEVs).
“One of the reasons that Toyota is starting with HEVs is because it wants to introduce solid-state batteries to the market as soon as possible, gain customer feedback and continue to evolve them, Maeda said. “Rather than building a large-scale production line for BEVs, which require a large number of batteries, it is better to start with HEVs, which have smaller batteries and a development process with which Toyota is familiar. This would allow solid-state batteries to be introduced to the market faster, as well as enable improvement of the manufacturing technology for them.”
Pushing Ahead with Silicon
Graphite (carbon) mostly has served as the industry's choice for anode material during lithium-ion batteries’ initial volume-production phases. But attention is shifting to anode material with improved characteristics; silicon and lithium metal are front-runners and silicon – currently used in small ratios in the anode of some production-battery lithium-ion chemistries – remains the focus of new and emerging research efforts and various startup companies.
Late 2021 saw the announcement of new research from the University of California at San Diego (UCSD) pairing solid-state electrolyte with an all-silicon anode to create what the scientists are calling “a silicon all-solid-state battery.” As reported by UCSD's News Center, the work resulted in a “laboratory-scale full cell that delivers 500 charge and discharge cycles with 80 percent capacity retention at room temperature, which represents exciting progress for both the silicon anode and solid-state battery communities.”
Although silicon can deliver up to 10 times the storage capacity of a graphite anode, lithium-ion batteries with silicon added to the anode to boost energy density see impact on the number of charge cycles and energy retention. Much of those performance compromises is due to the interaction of silicon anodes and the liquid electrolytes used in all current production lithium-ion batteries. “For silicon anodes, we know that one of the big issues is the liquid electrolyte interface instability,” said UCSD nanoengineering professor Shirley Meng, the corresponding author on a paper about the research published in the journal Science. “We needed a totally different approach,” Meng, Director of the Institute for Materials Discovery and Design UCSD, told the UCSD News Center.
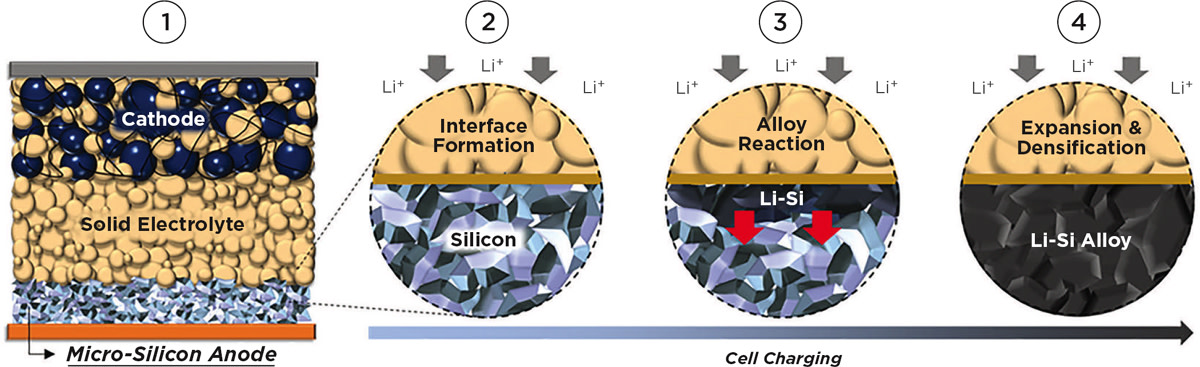
The UCSD researchers reported that they eliminated the carbon and the binders usually employed with all-silicon anodes. They also looked to micro-silicon, which is not as highly processed, and thus less-expensive, than nano-silicon typically used for anodes. The micro-silicon anode then was teamed with a sulfide-based solid electrolyte that experiments demonstrated is extremely stable for use with all-silicon anodes. By totally eliminating carbon in the anode, the team markedly reduced the interfacial contact (and corresponding performance-reducing effects) with the solid electrolyte, avoiding continuous capacity loss common with liquid-based electrolytes.
“The solid-state silicon approach overcomes many limitations in conventional batteries. It presents exciting opportunities for us to meet market demands for higher volumetric energy, lowered costs, and safer batteries especially for grid energy storage,” said Darren H. S. Tan, the first author of the Science paper. Tan also is the CEO and co-founder of a startup, UNIGRID Battery, which has licensed the technology for the silicon all-solid-state battery.
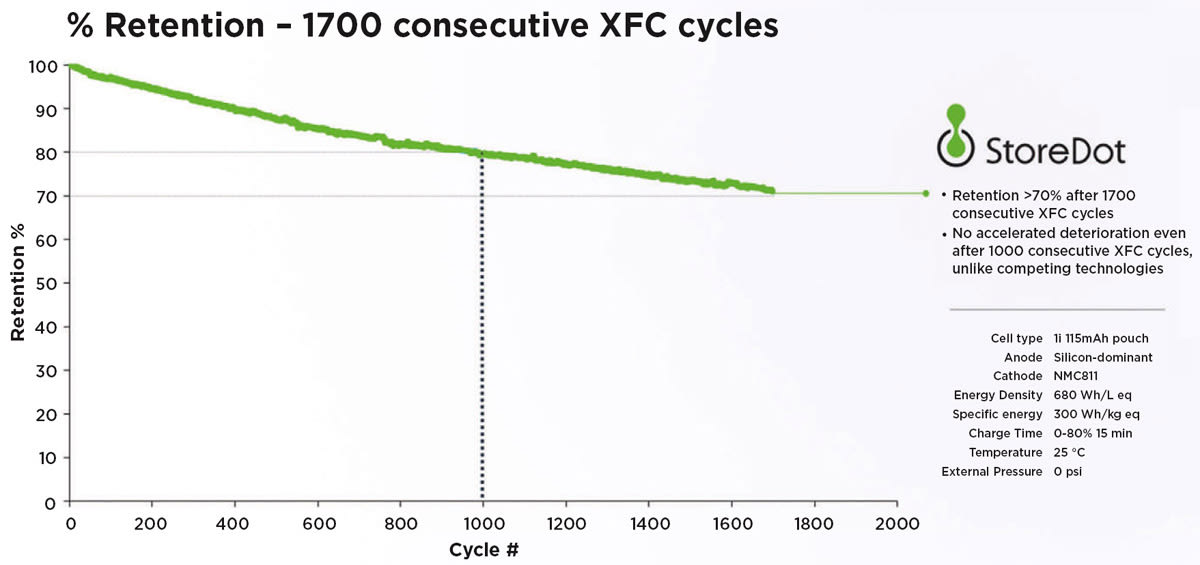
Meanwhile, any number of startups also continue to concentrate silicon-based solid-state battery designs. StoreDot, for one, aims to have a silicon-based solid-state battery (dubbed XED, or Extreme Energy Density) sometime in the 2028 timeframe. The company has said resulting energy density from silicon-based lithium-ion batteries can enable DC fast charging that delivers up to 25 miles (40 km) of range per minute while reducing long-term performance degradation. Another, Colorado's Solid Power, is developing its own silicon-intensive anode and pairs it with a proprietary sulfide-based solid electrolyte. BMW and Ford have invested in Solid Power and BMW has intimated it targets a mid-2020s launch for EVs using solid-state technology.
Another solid-state battery developer, QuantumScape, has said graphite anodes are responsible for many of the limitations of contemporary lithium-ion batteries. QuantumScape, which since 2015 has collaborated with the Volkswagen Group, is hoping for energy density of “close to” 1000 Wh/liter for its novel battery design that is manufactured without an anode – charging the cell causes an anode of pure lithium metal to form.
This article was written by Bill Visnic, Editorial Director, Mobility Media, SAE International. For more info visit here .


