Decarbonizing transportation is key for meeting U.S. greenhouse gas reduction targets because moving people and goods is the largest direct source of climate-altering emissions. Consequently, analysts predict a coming surge in electric vehicle sales.

According to BloombergNEF, for example, worldwide passenger electric vehicle sales will rise from 3.1 million in 2020 to 14 million in 2025. By 2040, BloombergNEF analysts say as much as 90 percent of all new passenger vehicle sales will be electric. A 2022 survey by AutoPacific found three out of four American consumers considered electric vehicles the way of the future.
Those electric cars, trucks, buses, and other vehicles need batteries. Although other options are being considered, the leading technology today to power those vehicles is lithium-ion (Li-ion) batteries. However, putting tens of millions of new electric vehicles on the road every year will create a critical materials supply challenge.
“If we convert a significant fraction of our fleet, we could start running into supply problems, mostly cobalt, nickel and lithium,” said Tedd Lister, an INL staff scientist. He is also a member of a research team that developed a new Li-ion battery material recycling technology that is more efficient and environmentally friendly than current methods.
Tighter Recycling Loop
Battery recycling is vital because one solution to the looming supply issue would be a closed-loop setup, with batteries processed at end-of-life to recover cobalt, lithium, and other critical materials that can then be used to make new batteries.
Significant recycling already takes place. In 2018, 20 companies worldwide recycled just under 100,000 metric tons of Li-ion batteries, about half of the global volume of batteries disposed that year. Recovering materials can involve direct recycling, pyrometallurgy, and/or hydrometallurgy.
Direct recycling attempts to employ a tighter recycling loop and retain more manufacturing value. A major target is the rejuvenation of the cathode material, so that it can be directly reused in a battery of the same type. In practice, however, because battery formulations change rapidly and Li-ion batteries are designed to last a decade or longer, the value of this approach is limited. Another challenge is the need to segregate collected batteries by manufacturer and production time, since every manufacturer uses unique cathode material.
Pyrometallurgical processes can accept different kinds of batteries, using high temperatures (above 700 °C) in a furnace to smelt the battery into an alloy. Hydrometallurgical refining using acids and other chemicals then separates and recovers cobalt, nickel, and copper from the alloy, while the lithium is extracted from the waste slag. Because of the temperatures required, the recycling process can have a significant carbon footprint if fossil fuels provide the energy for the smelting.
One way to reduce the greenhouse gas emissions from Li-ion battery recycling is to use hydrometallurgy alone to leach out materials. Often this involves use of sulfuric acid and hydrogen peroxide because the combination is very effective; reported yields for the target metals are over 90 percent when the leaching is conducted at temperatures above 40 °C. Making the chemicals, though, has significant negative environmental impacts and presents safety risks, as does transporting and storing them.
An Electrochemical Boost
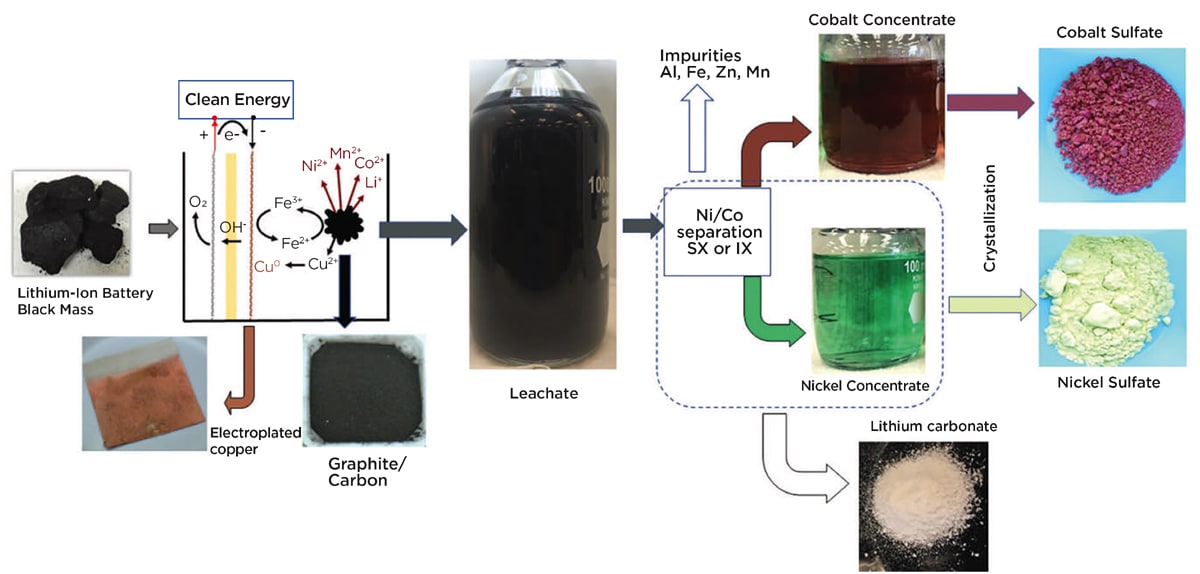
As described in the peer-reviewed scientific journal, Resources, Conservation and Recycling, an INL team investigated a different approach employing an electrically driven hydrometallurgical process. INL researchers developed an electrochemical-assisted leaching method that continuously regenerates Fe2+ in small concentrations. The Fe2+ reacts with the Li-ion battery cathode metals to promote their extraction into the aqueous phase. The team achieved near complete metal leaching from Li-ion battery “black mass”’ material recovered from shredded Li-ion batteries that contains the active battery components.
The electrochemical boost to the leaching process is an important component in achieving the goal of a greener Li-ion batteries recycling solution.
“Electrochemistry is the transformation of electrical energy into chemical bonds or chemical bonds into electrical energy” said Luis Diaz-Aldana, an INL electrochemical scientist and team member. “The vision that we had is that we can use this electricity as a green reagent. So that it can substitute for a significant amount of chemicals.”
He added that the electricity could come from a carbon-free source, such as a solar panel array, a wind farm, a hydroelectric dam, or a nuclear power plant. This would reduce total emissions associated with the new recycling process as compared to competing processes to an even greater degree.
The process has been scaled from the initial proof-of-principle demonstration to a system that can process over 0.5 kg/day. From an industrial battery recycler, the team obtained metal oxide black mass composed of the anode and cathode powder recovered from a mixture of different Li-ion batteries.
The black mass contained lithium, cobalt, manganese, nickel, and aluminum in observed formulations, such as LiCoO2, LiMnxCoyO2, LiNixMnyCo zO2, and LiNixCoxAlzO2. Along with these lithium-containing materials, more than 30 percent of the weight in the black mass was nonmetallic, including graphite from the anode and elsewhere, carbon material and polymeric bits of battery separators.

The INL researchers put the black mass on one side of the bipolar membrane in a sulfuric acid – iron sulfate solution, along with a stir magnet and a stainless-steel cathode mesh. They put water and a nickel anode on the other side of the membrane. They then operated the chamber at a -0.3 V cathode potential, causing the cobalt, lithium, manganese, and nickel to leach out of the cakes at efficiencies over 96 percent, a near total recovery of these critical materials from the shredded Li-ion batteries. The copper plated out on the electrode and the graphite also deposited in a form suitable for further processing.
This material extraction occurred at room temperature, at an estimated electrical consumption of 232.3 kWh per ton of black mass. The researchers calculated that using the electrochemical approach would cut the cost of chemicals by as much as 84 percent as compared to following the traditional sulfuric acid-hydrogen peroxide method. In addition to those cost savings, less chemical consumption would also mean less environmental impact from chemical production, storage, transport, and disposal.

Overall, the decrease in chemical consumption and room-temperature operation should shave about 80 percent off the cost of chemicals and energy, according to an analysis by the researchers. Optimization of the process could make those savings even greater, for both operating and capital costs.
The electrochemical process has been patented and research into complementary processes continues, Lister said. The output from the leaching process, for example, is a critical material-rich solution and the dissolved metals must be separated and converted into products suitable for manufacturing new cathodes. This is an area of active development, with considerable progress made toward the final goal of a sustainable commercial Li-ion battery recycling process. “We'll have a complete package we can show somebody,” he said.
This article is written by Hank Hogan, President, Hank Hogan Writing and Editorial, for Idaho National Laboratory (Idaho Falls, ID). For more info visit here .

