
Existing commercial battery technologies, which use liquid electrolytes and carbonaceous anodes, have certain drawbacks such as safety concerns, limited lifespan, and inadequate power density particularly at high temperatures.
A research team led by Professor Dong-Myeong Shin of the Department of Mechanical Engineering at the University of Hong Kong (HKU) has developed a new generation of lithium metal batteries, representing a significant advancement in the field. Their innovation centers on microcrack-free polymer electrolytes, integral to these batteries, which promise extended lifespan and enhanced safety at elevated temperatures.
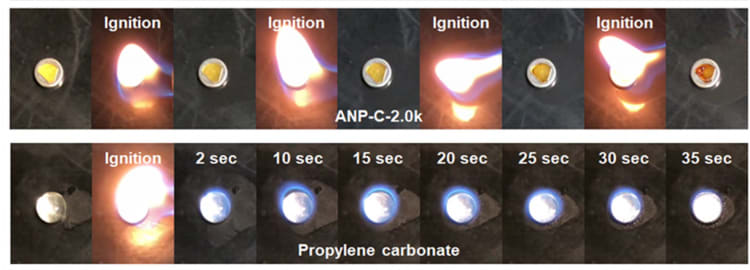
The microcrack-free polymer electrolytes developed by Shin's team are synthesized via a straightforward one-step click reaction, exhibiting notable attributes including resistance to dendrite growth and non-flammability, demonstrating a high electrochemical stability window up to 5 V, and an ionic conductivity of 3.1 × 10-5 S cm-1 at high temperatures.
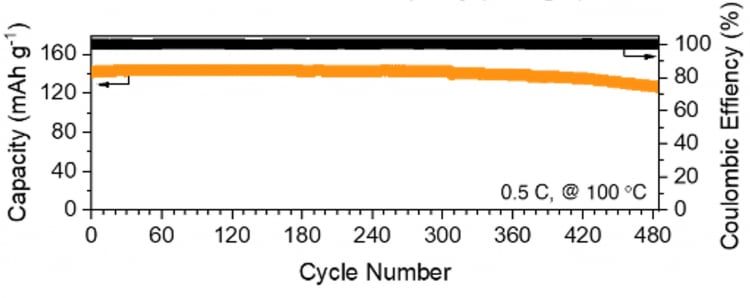
These enhancements are attributed to tethered borate anions within the microcrack-free membranes, which facilitate accelerated selective transport of Li+ ions and suppress dendrite formation. Ultimately, these anionic network polymer membranes enable lithium metal batteries to function as safe, long-cycling energy storage devices at high temperatures, maintaining 92.7 percent capacity retention and averaging 99.867 percent coulombic efficiency over 450 cycles at 100 °C. Normally, the cycling performance of conventional liquid electrolytes Li metal batteries is fewer than 10 cycles at high temperatures.
The breakthrough by the research team potentially paves the way for future advancements in anionic polymer electrolyte design for next-generation lithium batteries.
“We believe this innovation opens doors for new battery chemistries that can revolutionize rechargeable batteries for high-temperature applications, emphasizing safety and longevity,” said First Author Jingyi Gao, Ph.D.
"Apart from applications in high-temperature scenarios, the microcrack-free electrolyte membranes also have the potential to enable fast charging due to low overpotential. This capability could allow electric vehicles to recharge in the time it takes to drink a cup of coffee, marking a significant advancement towards a clean energy future," Shin added.
Here is an exclusive Tech Briefs interview, edited for length and clarity, with Shin.
Tech Briefs: What was the biggest technical challenge you faced while developing this new generation of lithium metal batteries?
Shin: The biggest technical challenge we faced was improving the ionic conductivity of the solid-state electrolyte while maintaining safety and stability. Solid-state electrolytes generally have lower ionic conductivity compared to traditional liquid electrolytes, which limits their performance in practical applications. Achieving a balance between high energy density, fast ion transport, and long-term cycle stability, especially under high-temperature conditions, required significant innovation in materials and design.
Tech Briefs: Can you explain in simple terms how it works?
Shin: Our battery uses a new all-solid-state polymer electrolyte instead of the liquid electrolytes found in most commercial batteries. Liquid electrolytes can be flammable and unsafe, especially at high temperatures. By switching to a solid-state polymer, which doesn’t contain any organic solvents and has various functional groups, we greatly reduce the risk of fire while also improving the battery's stability and lifespan, even under high-temperature conditions.
Tech Briefs: What are your next steps? Do you have plans for further research/work/etc.?
Shin: Our next steps involve adding more advanced features to the battery system, such as developing self-healing batteries. This would allow the battery to repair itself if damaged, further enhancing its safety and durability. By integrating self-healing capabilities, we aim to provide even greater protection and extend the battery's lifespan, especially in demanding environments. This continued research will help push the boundaries of battery technology for high-temperature applications.
Tech Briefs: You’re quoted in the article as saying, "Apart from applications in high-temperature scenarios, the microcrack-free electrolyte membranes also have the potential to enable fast charging due to low overpotential. This capability could allow electric vehicles to recharge in the time it takes to drink a cup of coffee, marking a significant advancement toward a clean energy future.” How far away from that do you estimate we are?
Shin: It's still a long and challenging journey because the ionic conductivity of solid-state batteries is currently lower than that of commercial liquid electrolyte batteries, which is a key factor in enabling fast charging. Achieving fast-charging capabilities with solid-state technology requires further breakthroughs, and it will take collective efforts from researchers around the world. Some companies are already planning to bring these advancements to production within the next 5 to 10 years, but there's still much work to be done.
Tech Briefs: I know the article was published recently, but do you have any updates you can share?
Shin: Since the article was published, we've made some exciting progress. We’re exploring new materials that could further enhance the battery’s efficiency and safety. While we're still in the research phase, these developments are promising and align with our goal of creating safer, longer-lasting batteries for high-temperature applications. We’re optimistic about the next steps and look forward to sharing more updates soon.
Tech Briefs: Do you have any advice for engineers/researchers aiming to bring their ideas to fruition, broadly speaking?
Shin: My advice is to keep trying and stay persistent. Research is a journey of constant trial and error. The path to achieving your final goal is often full of twists and turns, but it's important to view setbacks as learning opportunities. Adjusting your approach along the way is a natural part of the process, and persistence is key to turning ideas into reality.
Tech Briefs: Anything else you’d like to add?
Shin: I'd just like to emphasize the importance of collaboration in pushing innovation forward. Whether it's in battery research or any other field, breakthroughs often come from teamwork and the exchange of ideas across disciplines. We're excited about the progress we've made, but there's still so much more to explore. We're hopeful that by continuing to work together, we can make significant strides toward safer, more efficient energy solutions for the future.

