A method of making more-complex molecules from simpler ones has emerged as a by-product of an experimental study in outer-space atom/ surface collision physics. The subject of the study was the formation of CO2 molecules as a result of impingement of O atoms at controlled kinetic energies upon cold surfaces onto which CO molecules had been adsorbed. In this study, the O/CO system served as a laboratory model, not only for the formation of CO2 but also for the formation of other compounds through impingement of rapidly moving atoms upon molecules adsorbed on such cold interstellar surfaces as those of dust grains or comets. By contributing to the formation of increasingly complex molecules, including organic ones, this study and related other studies may eventually contribute to understanding of the origins of life.
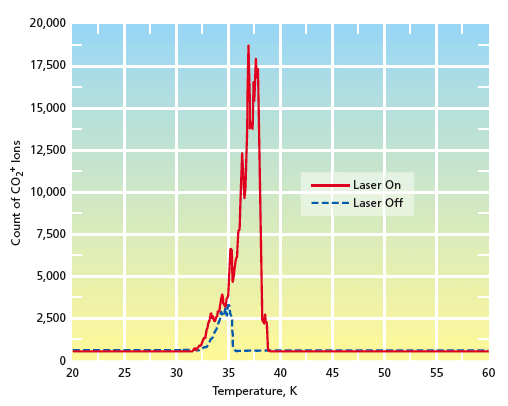
In the study, CO was adsorbed onto a cryo-cooled surface, then the surface was exposed to a beam of ground-state O atoms at a kinetic energy of 10 eV per atom. After an exposure time of 135 minutes, the surface was retracted from the O-atom beam into the field of view of a quadrupole mass spectrometer. The reaction products were desorbed by heating the cold surface according to a defined temperature-vs.-time schedule (temperature- programmed desorption). Desorbed molecules were ionized, then detected in the mass spectrometer. The temperature dependence of the CO2 peak in the mass-spectrometer readout (see figure) indicated that large quantities of CO2 were desorbed; this observation was taken to be evidence for the reaction O + CO(s) → CO2(s).
Generalizing the method used in this study, it may be possible, for example, to make simple and more-complex amines, even amino acids by reacting ice mixtures of CH4 and NH3 with superthermal O and H beams. In general, by choice of atomic projectiles, kinetic energies, atomic quantum states, surfaces, and exposure times, it may be possible to create new molecular species and stabilize them on the solid surfaces on which they were created.
This work was done by Brian Shortt, Ara Chutjian, and Otto Orient of Caltech for NASA's Jet Propulsion Laboratory.
NPO-41300
This Brief includes a Technical Support Package (TSP).

Making More-Complex Molecules Using Superthermal Atom/Molecule Collisions
(reference NPO-41300) is currently available for download from the TSP library.
Don't have an account?
Overview
The document titled "Making More-Complex Molecules Using Superthermal Atom/Molecule Collisions" (NPO-41300) from NASA's Jet Propulsion Laboratory (JPL) discusses a significant advancement in the understanding of atom-surface collision physics, particularly in the context of how simple atoms and molecules can react on cold surfaces to form more complex species. This research is motivated by the need to comprehend the chemical processes that occur in the universe, especially on celestial bodies like interstellar grains and comets, where atoms and small molecules may be abundantly adsorbed.
The primary focus of the study is on the reactions that occur when surface-adsorbed atoms or molecules interact with various external projectiles, including ions, neutral atoms, photons, and electrons. Notably, this research explores the previously unstudied interactions involving fast atoms and molecules. The team utilized the Fast-Atom Facility at JPL to generate a beam of fast oxygen atoms, which were directed at a cryo-cooled surface coated with carbon monoxide (CO) molecules.
After a 135-minute exposure to the fast oxygen atom beam, the surface was analyzed using a quadrupole mass spectrometer to identify the reaction products. The results revealed a significant production of carbon dioxide (CO2), providing clear evidence for the reaction O + CO → CO2. This finding marks the first laboratory observation of this recombination reaction using a well-defined beam of fast, ground-state oxygen atoms, highlighting the novelty and importance of the research.
The document emphasizes the broader implications of these findings, as understanding such surface reactions can shed light on the formation of complex organic molecules in space, which is crucial for astrobiology and the study of the origins of life. The research not only contributes to fundamental science but also has potential technological and commercial applications, as it is part of NASA's Commercial Technology Program aimed at disseminating aerospace-related developments.
For further inquiries or detailed information, the document provides contact details for the Innovative Technology Assets Management at JPL. Overall, this research represents a significant step forward in the field of astrochemistry and the understanding of molecular formation in extraterrestrial environments.

