As we collectively move away from fossil fuel power and work toward cleaner energy solutions, batteries are emerging as the preferred clean energy source. From portable consumer electronics to electric transportation to stationary energy storage, batteries are already powering many aspects of our modern world. Lithium-ion batteries have been used for nearly a century, but as we collectively look to do more with battery power, there’s increased demand for batteries with higher energy density and sustainable solutions across the value chain from raw materials to recycling.
According to the International Energy Agency (IEA), demand for batteries continues to grow, largely driven by the electric vehicle (EV) market and the need for higher performance batteries. Today, this rising EV demand is the greatest contributor to the increased demand for the mining and refining of critical metals, such as lithium and cobalt, and minerals, such nickel. To mitigate the demand for these raw materials and reduce environmental impact, scientists across the battery industry and academia are collaborating to progress research on innovative batteries technologies and introduce sustainable materials into battery development. However, understanding these often-delicate materials can be challenging, as scientists must use techniques that allow them to map samples at an atomic level without destroying the integrity of the material.
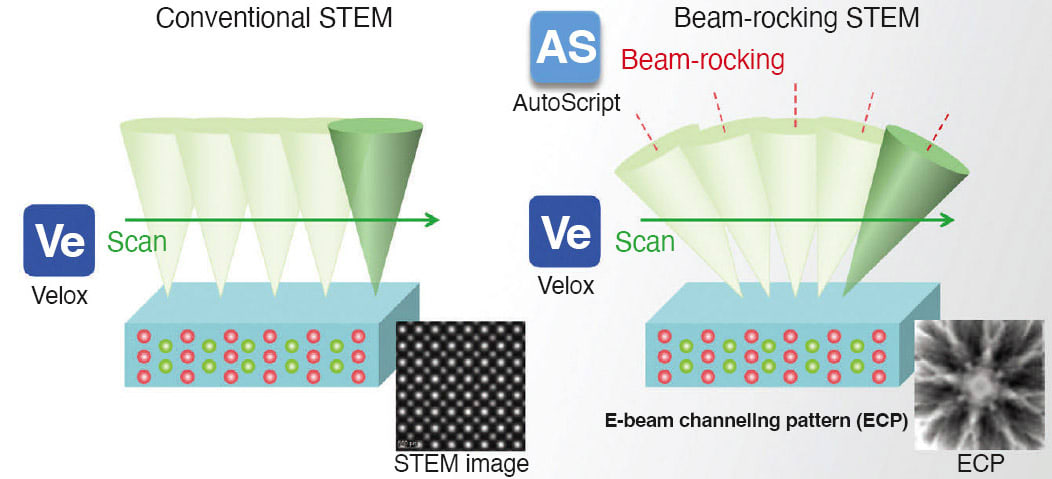
Balancing Precision and Preservation to Understand Delicate Materials
For many scientists working with beam-sensitive battery materials, mapping the trace elements at an atomic level is akin to searching for a needle in a haystack with a magnifying glass. They need to be able to characterize the material without triggering a combustion that would threaten the essence of the fragile matrix within the material.
A common challenge is using low electron beam dose imaging to locate these elusive atoms without damaging the material. In many instances, it’s a balancing act between precision and preservation as scientists venture into the microscopic realm to unlock the mysteries concealed within these intricate materials. However, there are innovative technologies, such as advanced electron microscopy, that can help with imaging and analysis of these delicate materials.
Atomic Level Insights for Higher Performance Batteries
A research team based at the Pacific Northwest National Laboratory was recently studying oxyfluoride disordered rock-salt (DRX) cathodes as a candidate for a next-generation battery material. According to their research, the DRX cathode shows great promise as a battery material for electric vehicles, as they could offer higher energy density, which means longer driving ranges, when compared to traditional cobalt cathodes. Since the DRX cathode is also cobalt-free, it could help to lessen the financial burden and environmental impact associated with sourcing many common battery materials.
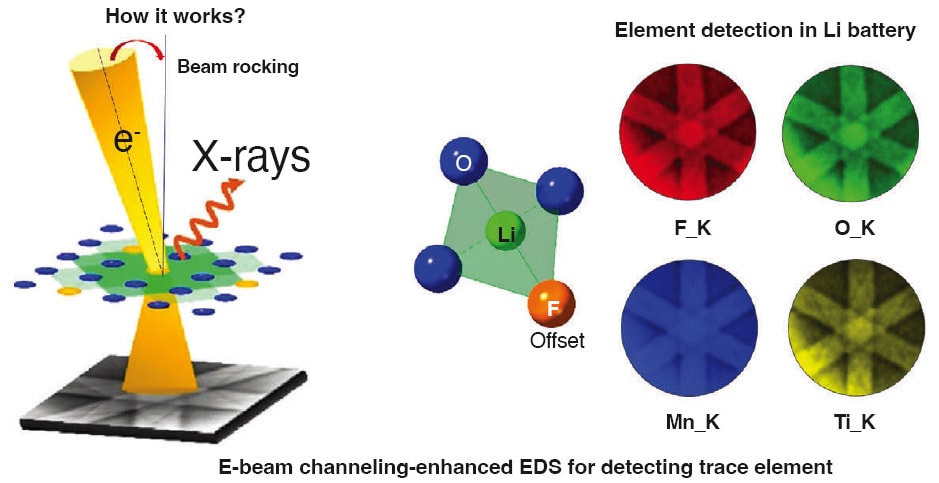
In an effort to enhance the capacity and lifespan of the DRX cathodes, the research team added a small amount of fluorine, which is difficult to accurately characterize at the atomic level without damaging the atoms. To overcome this challenge and progress their battery research, the team turned to transmission electron microscopy (TEM) techniques to achieve a scale that can clearly identify concentration, spatial distribution and electronic structure of the fluorine atoms within the DRX lattices.
Once they were able to view the atomic structure, the team discovered that replacing oxygen with fluorine in the DRX cathodes helps to reduce the oxygen loss and enhance redox capacity, making the cathode last longer. Traditional battery materials, such as lithium-ion, tend to lose oxygen too quickly which makes them less energy efficient over time. For example, the current battery chemistry used in EVs on the market today has an energy density of just under 500 watt-hours per kilogram (wh/kg), which is why the EVs have several hundred-mile limits before a full charge is required. But the research team found that the oxyfluoride DRX cathodes with fluorine have a much higher energy density with the potential to reach approximately 1,000 wh/kg.
In addition to the DRX cathodes contributing to more energy dense batteries, the new battery chemistry has the potential to offer flexible design options that enable lighter and more compact batteries, too. As automakers pledge fully electric fleets and major cities worldwide turn to battery power for public transportation, researchers in battery R&D are exploring alternative materials, such as DRX cathodes, for energy dense batteries that are safer, more cost-efficient, and sustainable.
Technologies Propelling Battery R&D
While the Pacific Northwest National Laboratory research team was able to use a combination of spectroscopic and microscopic techniques on a scanning TEM, there are several cutting-edge technologies and analytical solutions helping scientists research new materials for energy dense batteries. These include electron microscopy, spectroscopy, inductively coupled plasma mass spectrometry (ICP), Fourier transform infrared (FTIR), Raman, X-ray fluorescence (XRF) and chromatography. Using a suite of analytical tools can help researchers across academia and industry explore new materials and battery technologies to propel battery science toward cleaner energy solutions.
For EVs, many manufacturers are looking into solid-state electrolyte batteries (SSE) as they offer higher energy density than traditional lithium-ion batteries. SSE batteries are also a more environmentally friendly option because they can store more energy with less material. Sodium-based batteries are also being widely studied as the material is highly available and more sustainable, putting less pressure on supply chains and the environment.
While many of these new battery chemistries, including the DRX cathode, are still in the research phase and far from mass production, they could have the ability to offer higher performing battery technology that meets the demand for the EV market. To move toward commercialization, collaboration between academic institutions and the battery industry is vital to scale up new battery technologies.
Paving the Way for More Sustainable Batteries
Regardless of material, researchers need innovative technologies that allow them to analyze delicate materials from an atomic-level perspective to better understand the complexities and structural details of these new chemistries and translate lab findings into real-world applications. It’s also imperative that scientists, engineers and manufacturers work together to create a circular economy for sustainability within the battery industry. This combination of collaboration, advanced techniques and modern technologies will help to guide the design of next-generation batteries that are longer-lasting, safer and more environmentally friendly.
This article was written by Lin Jiang, Staff Scientist, Research & Development, Thermo Fisher Scientific (Waltham, MA). For more information, visit here .

