Many of the devices that make modern life convenient and efficient rely on rechargeable batteries. Lithium-ion batteries, one of the more common types used, are low cost and work at a high operating voltage, which makes them ideal for many electronic devices and electric vehicles. However, they have recurring issues with declining performance over repeated use and there are rising concerns about the safety of using these batteries as they age.
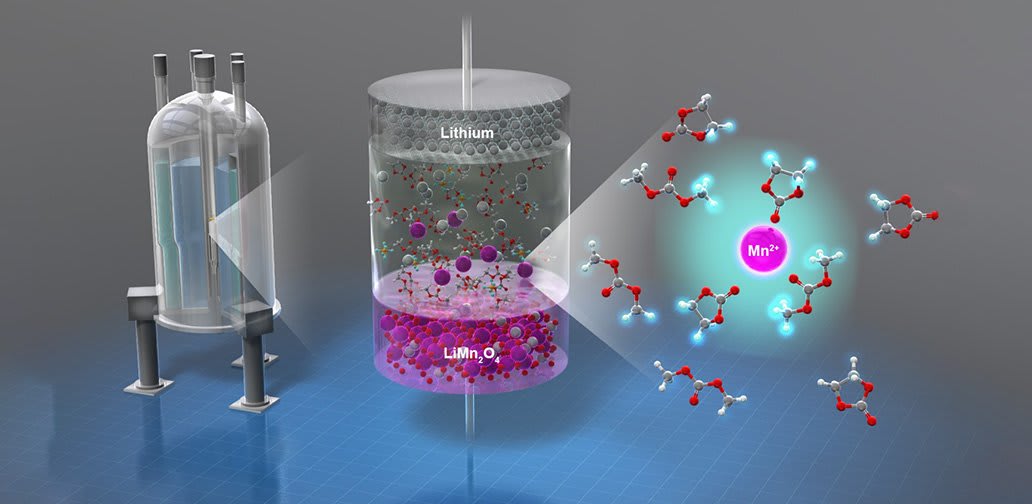
One of the causes of this decline in performance is the dissolution of the metal ion in the cathode into the electrolyte in the battery. However, it has been difficult to study this process as the amounts in the dissolution are very small. Consequently, to understand what is happening in the battery at the cathode, researchers need to know where, when and how much dissolution is occurring before they can address the problem.
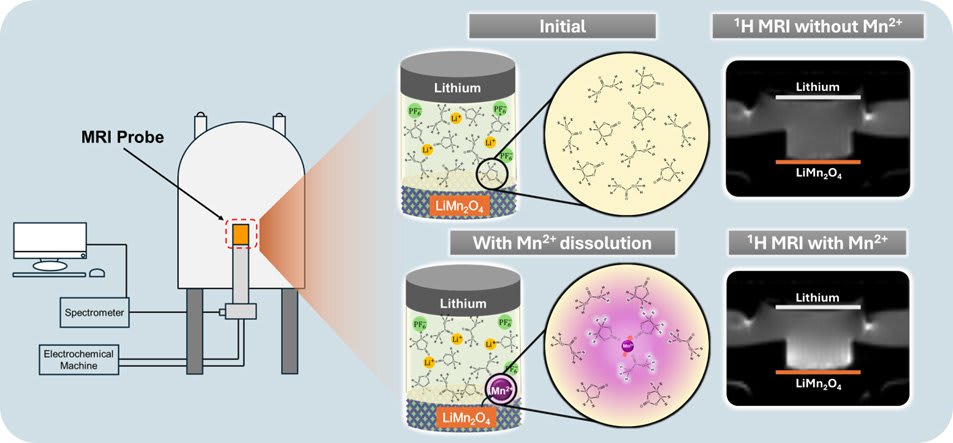
Researchers at Tohoku University have been working on a method to detect and investigate the dissolution of the metal ion in the cathode. Using nuclear magnetic resonance imaging (MRI), they were able to directly observe the dissolution in real time.
The results of their research were published in Communications Materials.
Here is an exclusive Tech Briefs interview, edited for length and clarity, with Professor Junichi Kawamura; Dorai Arun Kumar, Ph.D.; Reiji Takekawa, Ph.D; and Nithya Hellar, a researcher at Tohoku University.
Tech Briefs: What was the biggest technical challenge you faced while developing this method to detect and investigate the dissolution of the metal ion in the cathode?
Researchers: At first, we intended to detect a valence change in LiMn2O4 cathode near the surface. But an unexpected strong change in the MRI signal intensity was observed at a high voltage region, which was finally attributed to the manganese dissolution after detailed investigations and discussions. When we focused on the manganese dissolution problem, there were some technical difficulties that needed to be overcome.
The biggest challenges we faced were:
- To rectify the artifacts generated while using metal electrodes and positioning them inside the NMR instrument.
- Unexpected electrochemical turbulence was observed in the MRI image during the charge-discharge process, especially while using a low viscosity electrolyte. It had to be suppressed to observe the quantitative picture, although the phenomena itself are very interesting (Additional research is underway).
- The electrochemical reaction and the metal ion dissolution take place at a very fast time scale. Therefore, to get detailed information about when, where, and how the dissolution is taking place, we had to acquire the MRI image at the exactly right moment in time. Optimizing the acquisition time and the resolution of the MRI image was a challenge.
Tech Briefs: What was the catalyst for this project?
Researchers: In the case of LIBs, the metal ion dissolution from the LiMn2O4 based cathodes is a serious problem when considering long term applications. Most of the work on metal ion dissolution is done in ex-situ analysis, where only quantitative information is gathered. However, in the case of LMO based cathodes, there are several reactions that lead to metal ion dissolution such as electrolyte oxidation, side reactions due to impurities in the electrolyte, and structural deformation. Therefore, identifying the origin of metal ion dissolution during operando conditions, will contribute to the development and modification of the electrode and electrolyte materials to suppress the metal ion dissolution.
Tech Briefs: Can you explain in simple terms how it works please?
Researchers: Transition metal ions (e.g. Mn, Co, Fe etc.) often have unpaired electrons in their outer orbitals and show paramagnetic behavior. The electron magnetic moment is ~600 times stronger than the nuclear magnetic moment of the proton, which strongly affects the proton NMR and enhances the MRI image. This effect is well known in medical MRI technique, but we are the first to use this principle to detect the transition metal dissolution problem in lithium-ion batteries.
Tech Briefs: Do you have any plans for further research/work/etc.?
Researchers: Most of the electrochemical energy storage devices work on the principle of reversible oxidation and reduction of the electrodes, where a transition metal ion changes its valency during operation. The technology developed in this study can be used for highly sensitive detection of various side reactions in battery reactions. The results of this research could be an important tool to support and accelerate the speed of research in material identification. In fact, our group has already applied this technique to lithium-sulfur batteries, which are expected to be a next-generation high-energy battery. We have reported that the MRI signal of a lithium-sulfur battery is enhanced in the same way as sulfur (S) dissolution due to the formation of sulfur radicals.1
Tech Briefs: Is there anything else you’d like to add that I didn’t touch upon?
Researchers: While we were searching for applications of this technique, the German group (MEET) proposed a new electrolyte that might suppress the manganese dissolution. We started an international collaboration to check the new electrolyte using this technique, which gives us good evidence for the technique’s usefulness.
Tech Briefs: Do you have any advice for researchers aiming to bring their ideas to fruition?
Researchers: The first idea may sometimes be incorrect, leading to unexpected results and outcomes. But don't be disappointed and disheartened; this is the perfect moment to initiate new science that will lead to groundbreaking discoveries.
1"Visualization of polysulfide dissolution in lithium-sulfur batteries using in-situ NMR microimaging," Arunkumar Dorai, Junichi Kawamura, Takahisa Omata, Electrochemistry Communications,141(2022)107360.

