An improved method of synthesis of crystalline tin monoxide (SnO) powder for use in anodes of lithium-ion electrochemical power cells has been developed. The carbon used in anodes of Li-ion cells is relatively expensive and difficult to produce, and SnO has been found potentially useful as an alternative anode material. One major disadvantage of the method heretofore followed in producing commercial crystalline SnO powder is that it involves heating. The improved method does not involve heating and thus offers the potential to reduce the cost, time, consumption of energy, and risk of contamination in the production of crystalline SnO powder. Fortuitously, it turns out that the improved method also yields powder with greater specific surface area, making it possible to fabricate electrodes with reversible charge/discharge capacities, for a fixed rate, greater than those of electrodes made from commercial crystalline SnO powder.
In the commercial method, synthesis begins with the dissolution of metal-ion-containing salt in distilled water. The resulting solution is added dropwise into a basic (e.g., NaOH) solution, where, typically, particles of amorphous metal hydroxide precipitate. The precipitate particles are washed, filtered, and dried in air. Next, the precipitate particles are heated in a crucible in air to a temperature typically above 200 °C to transform them from amorphous metal hydroxide to crystalline tin monoxide.
The improved method is derived from the commercial method but differs in key respects. Instead of starting by completely dissolving metal-ion-containing salt, one starts with an insoluble combination of SnCl2 in water to form SnxOyHz compounds. The tin-bearing solution obtained by hydrolysis in the improved method is acidic. The tin-bearing acidic solution is added dropwise to a basic solution (NaOH or NH4OH). However, whereas the pH of the basic solution is allowed to fall to about 10 or 11 as the acidic solution is added dropwise in the commercial method, the pH is not allowed to fall in the improved method; instead, NaOH is added continuously to maintain the pH of the basic solution at 14 during the dropwise addition of the acidic tin-bearing solution. A white gel precipitate is formed and, after about 45 minutes, becomes transformed into a black precipitate comprising aggregated crystalline SnO powder. The precipitate is washed, filtered, and dried as in the commercial method, but unlike in the commercial method, no further processing (that is, no heating) is needed.
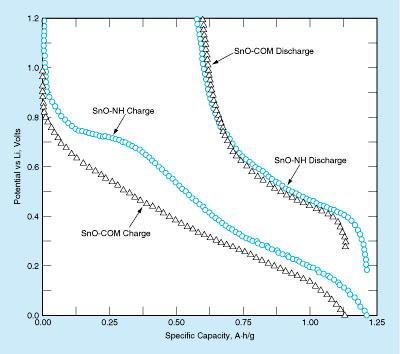
The electrochemical performance of crystalline SnO powder synthesized by the commercial method (SnO-COM) and of SnO powder synthesized by the improved method with no heating (SnO-NH) were measured during charging and discharging of half cells. Each half cell included a nickel-mesh-supported lithium foil that served as both an anode and a reference electrode, plus a cathode fabricated by spraying a mixture of SnO powder, a poly (vinylidene fluoride) binder, and carbon black onto a copper foil substrate. The electrodes in each cell were separated by 50-μm-thick poly-propylene membranes. The cells were filled with an electrolyte mixture of LiPF6, ethylene carbonate, and dimethyl carbonate.
The figure presents titration curves from charging and discharging of the half cells between a charge cutoff potential of 1.2 V and a discharge cutoff potential of 0 V. These curves indicate a greater specific charge capacity for the SnO-NH electrode than for the SnO-COM electrode. The increase in specific capacity can be attributed to greater specific surface area (0.871 m2/g for SnO-NH vs. 0.297 m2/g for SnO-COM). This effect increases the accessible electrode surface area and ease of incorporation and extraction of Li ions.
This work was done by Chen-Kuo Huang and Subbarao Surampudi of Caltech and Jeffrey Sakamoto and Jeffrey Wolfenstine of UC Irvine for NASA's Jet Propulsion Laboratory. NPO-20355
This Brief includes a Technical Support Package (TSP).

Improved synthesis of SnO powder for lithium-ion power cells
(reference NPO20355) is currently available for download from the TSP library.
Don't have an account?
Overview
The document discusses an improved method for synthesizing crystalline tin monoxide (SnO) powder, which is significant for lithium-ion power cells. The traditional commercial method involves dissolving stannous chloride (SnCl2) in distilled water, followed by a dropwise addition to a basic solution (like NaOH), resulting in the precipitation of amorphous metal hydroxide. This precipitate is then heated to convert it into crystalline SnO. However, the new method innovates on this process by starting with an insoluble combination of SnCl2 in water to form Sn₄OH compounds, leading to an acidic tin-bearing solution.
In the improved method, the acidic solution is added dropwise to a basic solution while continuously maintaining the pH at 14, unlike the commercial method where the pH is allowed to drop to around 10 or 11. This adjustment results in a white gel that transforms into a black aggregate after about 45 minutes, which is confirmed to be crystalline SnO through X-ray diffraction (XRD) analysis. The document highlights that the new synthesis method yields a powder with a greater specific surface area, enabling electrodes to achieve reversible charge/discharge capacities that are approximately 20% higher than those made from commercially available crystalline SnO powder.
The document also includes experimental details, such as the specific quantities of chemicals used and the conditions under which the synthesis occurs. It emphasizes the importance of maintaining a high pH during the synthesis process to ensure the desired transformation of the gel into crystalline SnO. Additionally, it notes that attempts to produce the black aggregate at lower pH levels were unsuccessful, underscoring the significance of the improved method.
Overall, this advancement in SnO powder synthesis not only enhances the performance of lithium-ion batteries but also simplifies the manufacturing process, potentially leading to cost reductions and increased efficiency in energy storage technologies. The findings are relevant for researchers and industries focused on battery technology and materials science, indicating a promising direction for future developments in energy storage solutions.

