Experiments have shown that improved hydride-forming negative electrodes for rechargeable nickel/metal hydride (Ni/MH) electrochemical cells can be made by substituting Sn for some of the Ni in LaNi5. Since the year 1988, it has been known that partial substitution of Sn for some of the Ni in LaNi5 slows the deterioration of reversible hydrogen-storage capacity and lowers the operating pressure for gas-phase cycling. However, prior to these experiments, the effects of the partial substitution on charge/discharge capacities on Ni/MH cells and on retention of their charge/discharge capacities during electrochemical reactions was not known.

Cyclic lifetime is an important issue in the technology of Ni/MH cells. Hydride-forming electrodes made of LaNi5 undergo severe deterioration of charge/discharge capacities during charge/discharge cycling and thus have short cycle lives. It has been known since 1984 that the deterioration can be slowed by substituting small amounts of other elements for both La and Ni. Unfortunately, early attempts to prolong cycle lives in this way produced undesired side effects in the form of decreases in hydrogen-absorption capacities, slow kinetics, and prolongation of activation intervals (intervals of initial charge/discharge cycling needed to achieve full capacities).
The experiments showed that the electrochemical (charge/discharge) capacity of LaNi5 -xSnx increases significantly with x up to about 0.25 (see Figure 1). The maximum discharge capacity observed in the experiments was slightly more than 300 mA·h/g - an impressive value for an alloy of this type and greater than the capacities (250 to 275 mA·h/g) of some of the misch-metal-based hydride-forming alloys that are being processed for electrodes in Japan and China. The substitution of Sn for some of the Ni results in low plateau pressures, with consequent low operating pressures and low self-discharge in alkaline rechargeable batteries.
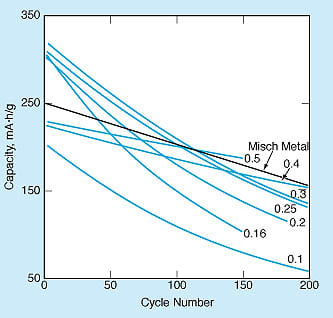
The performances of LaNi5 -xSnx alloys during charge/discharge cycling were evaluated in 250 mA·h, negative limited, prismatic laboratory cells (see Figure 2). The cells were designed in the MH-limited configuration to gain understanding of the life-limiting mechanisms of MH electrodes and to carry out a comparative evaluation of their cyclic lifetimes. Initial capacities were found to increase with x up to about 0.3 and then decrease with x beyond 0.3. After 100 full-capacity charge/discharge cycles, specimens with x = 0.25 and x = 0.3 exhibited capacities in excess of 200 mA·h/g - comparable to those of the best misch-metal-based alloys previously evaluated under identical conditions.
The capacities retained after 200 charge/discharge cycles were found to increase with x. Long activation intervals (30 charge/discharge cycles) were found to be necessary to achieve full capacities in the specimens with x ≥0.4, but this is a relatively minor disadvantage in that after extensive charge/discharge cycling, these specimens emerged as the ones that retained the greatest capacities. These alloys with highest concentrations of Sn look promising for use at high temperatures, where the plateau pressures of other alloys are too high.
This work was done by Ratnakumar Bugga, Subbarao Surampudi, Brent Fultz, Charles K. Witham, Robert C. Bowman, Jr., and Adrian Hightower of Caltech for NASA's Jet Propulsion Laboratory. For further information, access the Technical Support Package (TSP) free on-line at www.techbriefs.com under the Materials category.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to
Technology Reporting Office
JPL
Mail Stop 122-116
4800 Oak Grove Drive
Pasadena, CA 91109
(818) 354-2240
Refer to NPO-19805, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

LaNi5-xSnx electrodes for Ni/MH electrochemical cells
(reference NPO19805) is currently available for download from the TSP library.
Don't have an account?
Overview
The document is a NASA Technical Support Package detailing research on LaNi5-xSnx alloys for use in nickel/metal hydride (Ni/MH) rechargeable batteries. The introduction emphasizes the growing popularity of metal hydride materials in electrochemical devices, particularly as replacements for cadmium in nickel-cadmium (Ni-Cd) batteries. The advantages of Ni-MH batteries include higher specific energy, energy density, longer cycle life, and improved environmental compatibility, while maintaining fast charge and discharge capabilities.
A significant challenge identified in the document is the cyclic lifetime of metal hydride electrodes, particularly those based on LaNi5. These electrodes experience a notable decline in energy storage capacity during charge-discharge cycles. Previous studies have shown that substituting small amounts of other elements for nickel and lanthanum can mitigate this degradation. The document references evaluations by Willems and Sakai, which indicate that while these substitutions can enhance cycle lifetime, they may also lead to decreased hydrogen absorption capacity and slower kinetics.
The research presented in the document, conducted by a team from Caltech for NASA’s Jet Propulsion Laboratory, focuses on the effects of varying the concentration of tin (Sn) in the LaNi5 alloy. The findings reveal that the capacity of the alloys increases with the concentration of Sn up to approximately 0.3, after which it begins to decline. Notably, specimens with Sn concentrations of 0.25 and 0.3 exhibited capacities exceeding 200 mA·h/g after 100 full-capacity charge/discharge cycles, comparable to the best mischmetal-based alloys tested under similar conditions.
The document also highlights that the capacities retained after 200 charge/discharge cycles improve with increasing Sn concentration. However, specimens with Sn concentrations greater than 0.4 require longer activation intervals (about 30 cycles) to achieve full capacities. Despite this minor disadvantage, these higher Sn concentration alloys ultimately demonstrate superior capacity retention after extensive cycling.
In conclusion, the research indicates that LaNi5-xSnx alloys, particularly those with higher Sn concentrations, show promise for high-temperature applications in rechargeable batteries, potentially leading to significant advancements in energy storage technology. The document serves as a technical report on the innovative work being done in this field, with implications for consumer electronics and beyond.

