Improved zinc anodes for silver/zinc and nickel/zinc rechargeable electrochemical cells have been invented. This invention will increase the usefulness and decrease cycle-life costs of Ag/Zn and Ni/Zn cells in NASA Space-Station-support applications, for which batteries with high energy densities and long cycle lives are needed; examples of these applications include extravehicular mobility unit (EMU) batteries, the EMU portable life-support subsystem (PLSS) backpack batteries, and batteries in portable tools and equipment for extravehicular activities (EVAs). Inasmuch as many of these portable tools and other items of equipment are modified versions of commercial items (portable tools, lights, cameras, recorders, camcorders, radios, communications equipment, cellular telephones, and medical equipment), this invention might also prove beneficial in numerous commercial applications. Similarly, it could offer benefits in military applications, other government applications, and other applications that involve batteries.
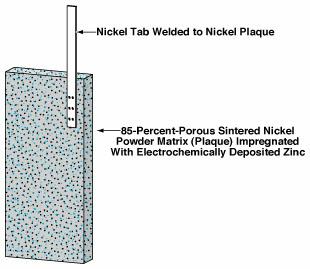
Two phenomena that limit the cycle lives of Ag/Zn and Ni/Zn secondary cells were unaddressed prior to this invention. These phenomena are (1) gradual changes in the shapes of zinc electrodes and (2) the deposition of zinc as dendrites during recharge.
- Regarding the shape changes: During charge/discharge cycling, the anodes become denser and lose active surface area. These changes cause progressive losses of capacity and thus of cycle life.
- Regarding the dendrites: These are sharp, needlelike crystals, which can penetrate cell separators and thereby cause internal short circuits.
Either phenomenon can lead to an uncomfortable, even a hazardous situation during a space flight. The invention maintains the integrity of a nickel anode, helping to prevent both of these phenomena. In so doing, it increases cycle life and thereby reduces the cost per cycle.
A typical conventional commercial zinc electrode contains a conductive grid made of perforated or expanded metal (typically copper) sheet. The invention involves a different approach: a zinc electrode according to the invention can be manufactured in a manner similar to that of making cadmium electrodes for aerospace cells. A porous sintered nickel powder matrix (plaque) is loaded with zinc by immersing the sinter in a zinc nitrate solution and electrochemically reducing the zinc. When the resulting anode is assembled into a battery, the form of the anode (see figure) is maintained by the nickel matrix. Even though nickel can give rise to excessive gassing by electrocatalyzing the decomposition of water, the use of nickel nevertheless confers an advantage by reducing the incidence of dendritic shorting and thereby extending cycle life.
Calculations have shown that the energy density of a cell is not impaired by substituting the zinc-loaded nickel-plaque anode for a conventional copper-grid-supported anode. Calculations have also shown that the plaque, when loaded with the same weight of zinc that would be included in a conventional anode, accommodates the increased volume of zinc oxide that is generated during discharge.
It has been suggested in some quarters that high-performance zinc anodes might be improved through use of a copper plaques of the proper porosity. However, there is no substantive evidence that this option would yield a greatly enhanced cycle life or address the shape-change and dendrite issues. Therefore, despite lower porosity (85 percent) of the nickel plaque relative to the 90-percent porosity of copper plaque, nickel plaque was chosen over copper plaque.
This work was done by John E. Casey of Lockheed Engineering & Sciences Co. for Johnson Space Center. For further information, access the Technical Support Package (TSP) free on-line at www.nasatech.com/tsp under the Materials category.
This invention has been patented by NASA (U.S. Patent No. 5,780,186). Inquiries concerning nonexclusive or exclusive license for its commercial development should be addressed to
the Patent Counsel,
Johnson Space Center,
(281) 483-0837.
Refer to MSC-22540.

