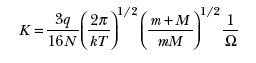A method is being developed for utilizing readings of an ion-mobility spectrometer (IMS) to estimate molecular masses of ions that have passed through the spectrometer. The method involves the use of (1) some feature-based descriptors of structures of molecules of interest and (2) reduced ion mobilities calculated from IMS readings as inputs to (3) a neural network. This development is part of a larger effort to enable the use of IMSs as relatively inexpensive, robust, lightweight instruments to identify, via molecular masses, individual compounds or groups of compounds (especially organic compounds) that may be present in specific environments or samples. Potential applications include detection of organic molecules as signs of life on remote planets, modeling and detection of biochemicals of interest in the pharmaceutical and agricultural industries, and detection of chemical and biological hazards in industrial, homeland security, and industrial settings.
The following background information is prerequisite to a meaningful summary of the present method.
- An IMS includes a drift tube that has length L and is filled with a drift gas (e.g., N2 or CO2) at a pressure, P, which could be atmospheric or any other suitable pressure. Mixed into the drift gas is a trace amount of ionized molecules from a sample or environment of interest. An electric potential (V) is applied between the ends of the drift tube.
- The mobility (K) of the ions is given by K ≡ L2/Vt, where t is the amount of time taken by the ions to drift along the tube from the inlet to a detector at the outlet.
The correlation among the mobility, the mass (m) of an ion, the mass (M) of a drift-gas molecule, and the cross section (Ω) for collisions between an ion and a drift-gas molecule is given by

where q is the fundamental unit of electric charge, N is the density of the drift-gas molecules, and k is Boltzmann’s constant.
- The reduced mobility (K0) is given by K0 ≡ KTSP/TPS, where TS denotes standard temperature (≈273 K) and PS is standard atmospheric pressure (represented by a mercury-barometer column height of 760 mm under normal Earth gravitation).
- In a previous study, it was found that there are some correlations between the molecular structure of each compound and the K0 value of ions of that compound in a given drift gas.
This concludes the background information.

The theoretical basis of the present developmental method can be summarized as the hypotheses that there could be a correlation among molecular structure, collision cross section, and molecular mass, such that it should be possible to estimate the mass of an ion by m = Φ(K0), where Φ is a nonlinear function to be determined, Ω^ is an estimated collision cross section that one strives to make as nearly equal as possible to the observed collision cross section. The estimated collision cross section is expressed as Ω^ = g(W,S), g is another nonlinear function to be determined, W is a vector of weights in a parameter space (e.g., a vector of neural-network weights), and S ≡ (d1,d2,d3,...) is a vector of feature-based numerical descriptors of the molecular structure. In this method, the applicable equations are not solved explicitly; rather, they are solved implicitly by means of a neural network (see figure). For each compound of interest, the inputs to the neural network are (1) a set of six feature-based descriptors extracted from a much larger set of molecular-structure descriptors by means of principal- component analysis and (2) K0 values for that compound in two different drift gases.
In a numerical-simulation test of the method, the neural network was trained by use of descriptors, K0 values, and molecular masses pertaining to 65 organic compounds, then interrogated by use of descriptors and K0 values pertaining to 10 other organic compounds. The molecular masses generated by the neural network were found to differ from the correct values by root-mean-square errors of no more than a few percent.
This work was done by Tuan Duong and Isik Kanik of Caltech for NASA’s Jet Propulsion Laboratory.
This Brief includes a Technical Support Package (TSP).

Utilizing Ion-Mobility Data To Estimate Molecular Masses
(reference NPO-44576) is currently available for download from the TSP library.
Don't have an account?
Overview
The document titled "Utilizing Ion-Mobility Data to Estimate Molecular Masses" (NPO-44576) from NASA's Jet Propulsion Laboratory presents a study focused on predicting molecular weights of chemical compounds using a feature-based descriptor approach. The research addresses the challenge of estimating molecular weights from ion-mobility data, which is crucial for understanding chemical structures and their implications for life.
The study involves the analysis of 75 chemical compounds, utilizing a total of 1664 descriptors to capture the essential characteristics of these compounds. To streamline the data, Principal Component Analysis (PCA) is employed, reducing the dimensionality from 1664 to just six feature-based descriptors. This reduction is significant as it retains 97% of the original data's information while simplifying the dataset, making it more manageable for neural network estimators.
The methodology includes training the neural network with 65 random sets of the chemical compounds and testing it with the remaining 10 sets. The research emphasizes the correlation between chemical structure and collision cross section, demonstrating that the feature-based approach offers clear advantages over traditional reduced descriptor techniques. The experimental simulations were conducted repeatedly in a random fashion over 100 iterations, reinforcing the reliability of the results.
The novelty of this work lies in its innovative use of second-order statistical techniques to derive meaningful descriptors that effectively represent the chemical compounds. This approach contrasts with existing methods that often rely on a limited selection of descriptors, which may not adequately capture the complexity of the chemical structures involved.
The document also highlights the broader implications of this research within the context of aerospace technology and its potential applications in various scientific and commercial fields. By improving the accuracy of molecular weight predictions, the findings could contribute to advancements in fields such as biochemistry, environmental science, and astrobiology, where understanding molecular characteristics is essential.
For further inquiries or detailed information, the document provides contact details for the Innovative Technology Assets Management at JPL, emphasizing the collaborative nature of NASA's research initiatives and their commitment to sharing technological advancements with the wider community.