A family of polymer/ carbon films has been developed for use as sensory films in electronic noses for detecting SO2 gas at concentrations as low as 1 part per million (ppm). Most previously reported SO2 sensors cannot detect SO2 at concentrations below tens of ppm; only a few can detect SO2 at 1 ppm. Most of the sensory materials used in those sensors (especially inorganic ones that include solid oxide electrolytes, metal oxides, and cadmium sulfide) must be used under relatively harsh conditions that include operation and regeneration at temperatures >100 °C. In contrast, the present films can be used to detect 1 ppm of SO2 at typical operating temperatures between 28 and 32 °C and can be regenerated at temperatures between 36 and 40 °C.
The basic concept of making sensing films from polymer/ carbon composites is not new. The novelty of the present family of polymer/ carbon composites lies in formulating the polymer components of these composites specifically to optimize their properties for detecting SO2. First- principles quantum- mechanical calculations of the energies of binding of SO2 molecules to various polymer functionalities are used as a guide for selecting polymers and understanding the role of polymer functionalities in sensing.
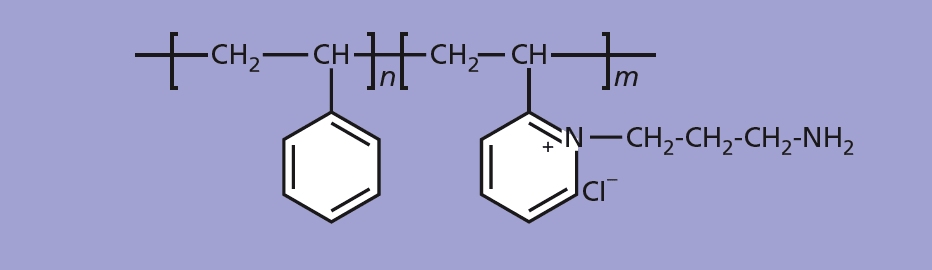
The polymer used in the polymer-carbon composite is a copolymer of styrene derivative units with vinyl pyridine or substituted vinyl pyridine derivative units (see figure). To make a substituted vinyl pyridine for use in synthesizing such a polymer, poly(2-vinyl pyridine) that has been dissolved in methanol is reacted with 3-chloropropylamine that has been dissolved in a solution of methanol. The methanol is then removed to obtain the copolymer. Later, the copolymer can be dissolved in an appropriate solvent with a suspension of carbon black to obtain a mixture that can be cast and then dried to obtain a sensory film.
This work was done by Margie Homer, Margaret Ryan, Shiao-Pin Yen, Adam Kisor, April Jewell, Abhijit Shevade, Kenneth Manatt, Charles Taylor, Mario Blanco, and William Goddard of Caltech for NASA’s Jet Propulsion Laboratory.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to:
Innovative Technology Assets Management
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109-8099
(818) 354-2240
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Refer to NPO-43761, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Special Polymer/Carbon Composite Films for Detecting SO2
(reference NPO-43761) is currently available for download from the TSP library.
Don't have an account?
Overview
The document discusses the development of special polymer/carbon composite films for detecting sulfur dioxide (SO₂) at low concentrations, specifically targeting detection levels as low as 1 ppm. This research is particularly relevant for NASA, as SO₂ can be a hazardous byproduct from leaking lithium-thionyl chloride batteries, posing health risks to astronauts in closed environments such as the International Space Station (ISS) and space shuttles.
Historically, most sensor materials for SO₂ detection have only been able to detect concentrations in the tens of parts per million (ppm) range and often require harsh operating conditions, such as temperatures exceeding 100 ºC. In contrast, the newly developed polymeric sensors operate effectively at milder temperatures (28-40 ºC) and demonstrate excellent regeneration capabilities, making them suitable for the specific requirements of NASA's Environmental Nose (ENose) system.
The innovative sensors are synthesized from copolymers that include combinations of vinyl pyridine, substituted vinyl pyridine, and styrene derivative units. The synthesis involves reacting vinyl pyridine with 3-chloropropylamine hydrochloride to create the necessary substitutions. The resulting polymers are then dissolved in a solvent with a carbon-black suspension, which is used to cast sensing films. These films have been successfully tested for their ability to detect SO₂ at concentrations as low as 1 ppm.
The theoretical foundation for selecting these polymer sensor materials is based on first principles calculations, specifically using quantum mechanics and Density Functional Theory (DFT) to determine binding energies for organic-SO₂ binary systems. The findings suggest that polymers containing amine functional groups, amides, aldehydes, and acids are promising candidates for effective SO₂ detection.
Overall, this research represents a significant advancement in sensor technology, providing a safer environment for astronauts by enabling the reliable detection of harmful gases at low concentrations. The development of these novel polymer-based sensors not only addresses the immediate needs of space missions but also has potential applications in various fields requiring gas detection in controlled environments.

