A class of developmental membrane electrolyte materials for methanol/air and hydrogen/air fuel cells is exemplified by a composite of (1) a melt-processable polymer [in particular, poly(vinylidene fluoride) (PVDF)] and (2) a solid proton conductor (in particular, cesium hydrogen sulfate). In comparison with previously tested membrane electrolyte materials, including those described in the two preceding articles, these developmental materials offer potential advantages of improved performance, lower cost, and greater amenability to manufacturing of fuel cells.
A principal limitation on the utility of the previously tested membrane electrolyte materials is that they must be hydrated to be able to conduct protons. This requirement translates to a maximum allowable operating temperature of about 90°C, and the presence of water in the polymer matrices undesirably gives rise to high permeability by methanol. It would be desirable to reduce permeability by methanol to increase cell performance and fuel-utilization efficiency, and it would be desirable to operate fuel cells at temperatures as high as 140°C to increase their tolerance to carbon monoxide from reformate streams. Therefore, what are needed are membrane materials that conduct protons in the absence of water.
In a composite material of the type undergoing development, the polymer serves as a matrix to support the solid proton conductor. In cesium hydrogen sulfate, proton conduction occurs by a mechanism that does not depend on water. At room temperature, the protons are in a bound state and so there is little or no proton conduction. However, as the temperature rises past 130°C and toward a value between 135 and 145°C, the cesium hydrogen sulfate undergoes a phase transition to a state in which the hydrogen ions have a significant amount of mobility; that is, the material becomes a proton conductor. The conductivity can be as high as 0.1 Ω–1cm–1 — of the order of the conductivities of the previously tested membrane electrolyte materials.
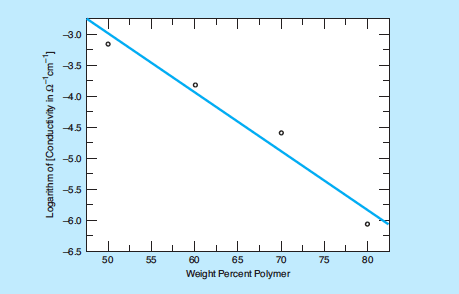
Some experimental polymer/solid electrolyte membranes have been fabricated by mixing PVDF and cesium hydrogen sulfate powders and pressing the mixtures in a die at temperatures between 160 and 190°C. Other experimental membranes were prepared by forming slurries of the powder mixtures in organic solvents, casting the slurries on a plate, allowing the slurries to dry, and then hot pressing the slurries. The figure shows the logarithms of electrical conductivities of these membranes as a function of composition. At the time of reporting the information for this article, it was anticipated that these membranes would be used to fabricate membrane/electrolyte assemblies for testing in fuel cells.
This work was done by Sekharipuram Narayanan, Sossina Haile, Dane Boysen, and Calum Chisholm of Caltech for NASA’s Jet Propulsion Laboratory.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to
Intellectual Property group
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109
(818) 354-2240
Refer to NPO-20645, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Improved Polymer/Solid-Electrolyte Membranes for Fuel Cells
(reference NPO-20645) is currently available for download from the TSP library.
Don't have an account?
Overview
The document discusses advancements in membrane electrolyte materials for fuel cells, specifically focusing on a composite of poly(vinylidene fluoride) (PVDF) and cesium hydrogen sulfate. These materials are being developed to enhance the performance of methanol/air and hydrogen/air fuel cells, addressing limitations of previously tested membrane electrolytes that require hydration for proton conduction. This hydration requirement restricts the maximum operating temperature to about 90 °C and leads to high methanol permeability, which negatively impacts fuel cell efficiency.
The innovative composite material aims to eliminate the need for water in proton conduction, allowing for higher operating temperatures, potentially up to 140 °C. This increase in temperature tolerance is crucial for improving the fuel cells' resistance to carbon monoxide from reformate streams, which can poison the catalysts used in fuel cells. The document highlights that cesium hydrogen sulfate conducts protons through a mechanism independent of water, with significant proton mobility achieved when temperatures exceed 130 °C.
Experimental efforts have involved fabricating polymer/solid electrolyte membranes by mixing PVDF and cesium hydrogen sulfate powders, followed by pressing or casting the mixtures. The resulting membranes have shown promising electrical conductivities, comparable to those of previously tested materials, with measurements taken at 130 °C.
The research was conducted by a team from Caltech for NASA’s Jet Propulsion Laboratory, emphasizing the potential for these new materials to improve fuel cell technology. The document also notes that the contractor retains title to the invention, and inquiries regarding commercial use should be directed to the Technology Reporting Office at JPL.
Overall, the development of these improved polymer/solid-electrolyte membranes represents a significant step forward in fuel cell technology, with the potential for enhanced performance, reduced costs, and greater manufacturing feasibility. The findings suggest that these materials could play a vital role in the future of clean energy solutions, particularly in applications where efficiency and durability are paramount.

