Instrumentation systems based on hairlike fiber-optic photochemical sensors have been proposed as minimally invasive means of detecting biochemicals associated with cancer and other diseases. The fiber-optic sensors could be mass-produced as inexpensive, disposable components. The sensory tip of a fiber-optic sensor would be injected through the patient's skin into subcutaneous tissue. A biosensing material on the sensory tip would bind or otherwise react with the biochemical(s) of interest [the analyte(s)] to produce a change in optical properties that would be measured by use of an external photonic analyzer. After use, a fiber-optic sensor could be simply removed by plucking it out with tweezers.
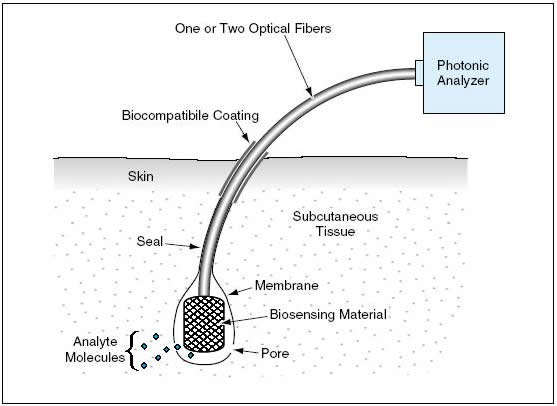

A fiber-optic sensor according to the proposal would be of the approximate size and shape of a human hair, and its sensory tip would resemble a follicle. Once inserted into a patient's subcutaneous tissue, the sensor would even more closely resemble a hair growing from a follicle (see Figure 1). The biosensing material on the sensory tip could consist of a chemical and/or cells cultured and modified for the purpose. The biosensing material would be contained within a membrane that would cover the tip. If the membrane were not permeable by an analyte, then it would be necessary to create pores in the membrane that would be large enough to allow analyte molecules to diffuse to the biosensing material, but not so large as to allow cells (if present as part of the biosensing material) to diffuse out. The end of the fiber-optic sensor opposite the sensory tip would be inserted in a fiberoptic socket in the photonic analyzer.
The basic concept of photonic detection of an analyte admits of the use of any of several alternative techniques. In one well-known technique, the biosensing material would be illuminated with light having the proper wavelength to excite fluorescence. The intensity and/or wavelength of the fluorescence would depend on the presence or absence of the bound analyte. In some cases, it may be desirable to use the same optical fiber to transmit the exciting light to the sensor and to transmit the fluorescence back to the photonic analyzer. The use of a single fiber would be appropriate if, for example, a brief excitation pulse of light could be expected to produce a longer-lived fluorescence that could be detected after the excitation pulse had been extinguished. In other cases, it may be necessary to use one optical fiber to transmit the excitation light to the biosensing material and another fiber to transmit the fluorescence back to the photonic analyzer. Alternatively or in addition to using fluorescence, it could be possible to measure the concentration of an analyte in terms of the amount of absorption of light of a particular wavelength from a broadband or spectrally modulated illumination.
Figure 2 illustrates a process that might be used to fabricate a two-fiber sensor according to the proposal. The two optical fibers would be bundled with a capillary tube at the end destined to become the sensory tip. The bundled end would be placed in a chamber, which would be partly evacuated and then back-filled with the vapor of a vapor-depositable material. As the vapor condensed and polymerized on the surface of the bundle, a droplet of biosensing material would be injected through the capillary tube. The droplet would become cooled rapidly by rapid evaporation in the partial vacuum. The cooling of the droplet would increase the rate of condensation of vapor and polymerization on the surface of the droplet, thereby causing the formation of the aforementioned membrane, which would be continuous with a tightly adherent coat over the contiguous optical fibers and capillary tube.
A suitable vapor-depositable material could be Parylene — a thermoplastic polymer made from poly-para-xylylene. Parylene is a highly biocompatible material that tends to discourage the adhesion and tracking of epithelial cells. Because Parylene exhibits little or no permeability by typical analytes that one might seek to detect, it would be necessary to create pores in the membrane. This could be done by, for example, burning holes by use of a tightly focused laser beam.
This work was done by Thomas George of Caltech and Gerald Loeb of the University of Southern California for NASA’s Jet Propulsion Laboratory. In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to:
Innovative Technology Assets Management
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109-8099
(818) 354-2240
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Refer to NPO-30651.
This Brief includes a Technical Support Package (TSP).

Hairlike Percutaneous Photochemical Sensors
(reference NPO-30651) is currently available for download from the TSP library.
Don't have an account?
Overview
The document outlines a novel approach to detecting and monitoring biologically important chemicals in the human body through a minimally invasive method. Developed at the Jet Propulsion Laboratory (JPL) under NASA's contract, the proposed technology involves the use of biosensors that can be injected into the body, specifically targeting well-vascularized areas like the scalp.
The core innovation lies in the biosensor's design, which utilizes cultured and modified cells, such as endothelial cells derived from the patient’s own body. These cells are engineered to express specific receptor molecules that bind to target analytes—biochemicals associated with diseases, including cancer. When the analyte binds to the receptor, it triggers a series of chemical changes within the cell, which can be detected using photonic methods. This detection process may involve changes in calcium channel activity, intracellular calcium levels, and fluorescence variations in a calcium-sensitive dye previously loaded into the cells.
The biosensor's small size, comparable to a human hair, allows for easy injection and placement under the skin. This feature not only minimizes discomfort for patients but also enables localized photonic detection of the biosensor's activity. Importantly, the design allows for the complete removal of the biosensor and its associated materials when they are no longer needed or if they cease to function.
The document emphasizes the novelty of this approach, highlighting its improvements over prior art, particularly in the context of chronic implantation techniques used in cosmetic applications, such as artificial hair. The integration of advanced biosensing technology with established methods of implantation represents a significant advancement in medical diagnostics.
Overall, this innovative biosensor technology has the potential to transform how diseases are detected and monitored, offering a more efficient, less invasive alternative to traditional diagnostic methods. The work reflects a commitment to advancing healthcare through cutting-edge research and development, with the ultimate goal of improving patient outcomes and facilitating early disease detection.

