Aliphatic esters have been found to be useful as electrolyte additives for improving the low-temperature performances of rechargeable lithium-ion electrochemical cells. The discovery of the beneficial effects of these additives was made during continuing research directed toward extending the lower limit of operating temperatures of these cells. Other aspects of this research have been described in the immediately preceding article and in prior NASA Tech Briefs articles referenced therein.
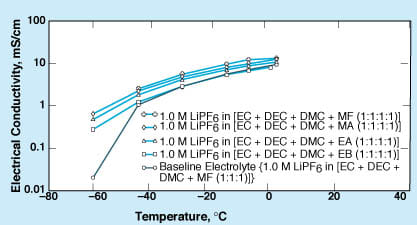
In experiments, the effects of aliphatic esters as additives were investigated with respect to a baseline optimal electrolyte formulation described in the noted previous articles; namely, a 1.0 M solution of LiPF6 in a solvent that consists of equal volume parts of ethylene carbonate (EC), dimethyl carbonate (DMC), and diethyl carbonate (DEC). In order of increasing molecular weight, the aliphatic esters investigated were methyl formate (MF), methyl acetate (MA), ethyl acetate (EA), ethyl propionate (EP), and ethyl butyrate (EB). These esters have freezing temperatures ranging from –73 to –98 °C — lower than the freezing temperatures of the carbonate solvents. They are fully miscible into the baseline electrolyte solution. In each case, the volume proportion of aliphatic ester incorporated into the electrolyte was equal to the volume proportion of one of the carbonate solvents.
The experiments included measurements of the temperature-dependent electrical conductivities of the ester-containing electrolytes, charge/discharge tests of lithium/graphite half cells containing these electrolytes, and ac-impedance and dc-micropolarization tests to determine the effects of electrolyte compositions on the electrochemical characteristics of films that formed on the graphite electrodes. The low-temperature electrical conductivities of the electrolytes were found to be increased by the addition of the esters, the greatest increase occurring in the cases of the esters of lowest molecular weight (see figure). However, the films formed in the presence of the higher-molecular-weight esters were found to be more stable and to exhibit better kinetics for lithium intercalation/de-intercalation, especially at lower temperatures. Taking both of these trends into account, it appears that the higher-molecular-weight esters are more promising as electrolyte additives.
This work was done by Marshall Smart, Ratnakumar Bugga, and Subbarao Surampudi of Caltech for NASA's Jet Propulsion Laboratory. For further information, access the Technical Support Package (TSP) free on-line at www.nasatech.com/tsp under the Materials category.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to
Technology Reporting Office
JPL
Mail Stop 122-116
4800 Oak Grove Drive
Pasadena, CA 91109
(818) 354-2240
Refer to NPO-20601, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Aliphatic Ester Electrolyte Additives for Lithium-Ion Cells
(reference NPO-20601) is currently available for download from the TSP library.
Don't have an account?
Overview
The document appears to be a New Technology Report (NTR) focused on the development and evaluation of organic ester additives for carbonate-based electrolytes in low-temperature lithium-ion cells. The research aims to address the challenges associated with the performance of lithium-ion batteries in cold environments, which can significantly impact their efficiency and reliability.
The report outlines the novelty of the work, emphasizing the unique properties of aliphatic ester additives that differentiate them from traditional carbonate solvents. These organic esters are noted for their lower freezing points, which can enhance the electrolyte's performance in low-temperature conditions. The motivation behind this research stems from the need to improve battery performance in various applications, particularly in environments where temperatures can drop significantly.
The technical disclosure section of the report details the problem that prompted the research, highlighting the limitations of existing electrolyte formulations in cold weather. It discusses how traditional carbonate-based electrolytes can become less effective at low temperatures, leading to reduced battery capacity and efficiency. The proposed solution involves the incorporation of organic ester additives, which not only lower the freezing point of the electrolyte but also improve its overall electrochemical stability.
The document likely includes experimental results demonstrating the effectiveness of these additives in enhancing the performance of lithium-ion cells at low temperatures. It may present data on the electrochemical properties of the modified electrolytes, including conductivity, viscosity, and overall battery performance metrics.
In conclusion, the report emphasizes the potential of organic ester additives to significantly improve the functionality of lithium-ion batteries in cold environments. This advancement could have far-reaching implications for various industries, including electric vehicles, portable electronics, and renewable energy storage systems. The findings suggest that further research and development in this area could lead to more robust and efficient battery technologies, ultimately contributing to the advancement of energy storage solutions.
Overall, the document serves as a valuable resource for researchers and industry professionals interested in battery technology and the ongoing efforts to enhance the performance of lithium-ion cells in challenging conditions.

