An improved cathode structure on a membrane/electrode assembly has been developed for a direct methanol fuel cell, in a continuing effort to realize practical power systems containing such fuel cells. This cathode structure is intended particularly to afford better cell performance at a low airflow rate.
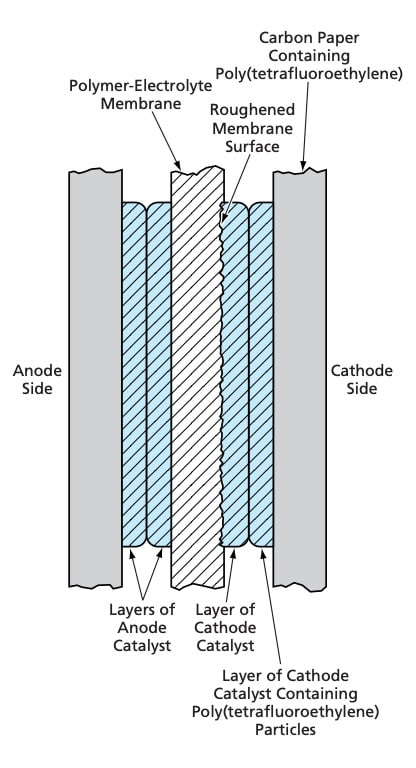
A membrane/electrode assembly of the type for which the improved cathode structure was developed (see Figure 1) is fabricated in a process that includes brush painting and spray coating of catalyst layers onto a polymer-electrolyte membrane and onto gas-diffusion backings that also act as current collectors. The aforementioned layers are then dried and hotpressed together. When completed, the membrane/electrode assembly contains (1) an anode containing a fine metal black of Pt/Ru alloy, (2) a membrane made of Nafion 117® or equivalent (a perfluorosulfonic acid-based hydrophilic, proton-conducting ion-exchange polymer), (3) a cathode structure (in the present case, the improved cathode structure described below), and (4) the electrically conductive gas-diffusion backing layers, which are made of Toray 060™ (or equivalent) carbon paper containing between 5 and 6 weight percent of poly(tetrafluoroethylene).
The need for an improved cathode structure arises for the following reasons: In the design and operation of a fuel-cell power system, the airflow rate is a critical parameter that determines the overall efficiency, cell voltage, and power density. It is desirable to operate at a low airflow rate in order to obtain thermal and water balance and to minimize the size and mass of the system. The performances of membrane/electrode assemblies of prior design are limited at low airflow rates. Methanol crossover increases the required airflow rate. Hence, one way to reduce the required airflow rate is to reduce the effect of methanol crossover. Improvement of the cathode structure — in particular, addition of hydrophobic particles to the cathode — has been demonstrated to mitigate the effects of crossover and decrease the airflow required.
The present improved cathode structure and the membrane/electrode assembly of which it is a part differ from prior such structures in the manner in which hydrophobic particles are distributed in the various layers and in the pretreatment of the membrane. The improved cathode is fabricated in a variant of the fabrication process summarized above. The major steps of this variant of the process as they affect the cathode are the following:
- The polymer-electrolyte membrane is roughened by use of a 600-grit-abrasive- coated paper.For a membrane area of 25 cm2, an ink comprising 0.18 g of Pt catalyst, 0.72 g of Nafion® (5 percent ionomer solution), and 0.40 g of water is applied to the abraded membrane surface by a paintbrush.
- Air is blown over the painted membrane surface to dry the ink.
- The gas-diffusion/ current-collecting carbon paper is brush-coated with an ink that contains the ingredients described for step 2, plus 0.035 g of poly(tetrafluoroethylene) particles.
- The membrane coated with catalyst layer is bonded to the gas-diffusion/ current-collecting carbon paper by use of heat and pressure.
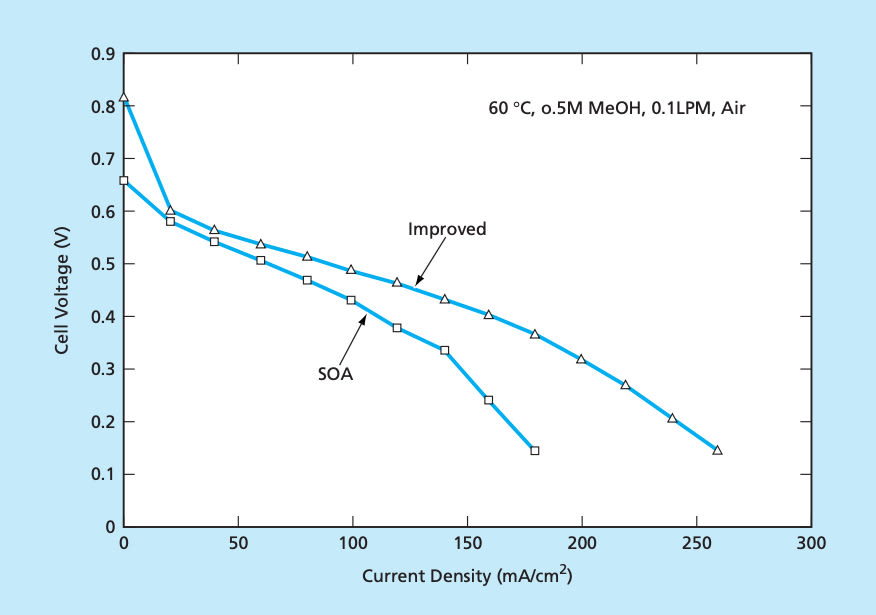
The performance of membrane electrode assemblies prepared by the new process is compared with those prepared by the state-of-art (SOA) process in Figure 2. The current density at 0.49 V has been raised from 70 mA/cm2 to 100 mA/cm2. This results in an increase in power density of 43 percent. At an applied current density of 100 mA/cm2, the cell voltage for the SOA cell and improved cell are 0.43 and 0.49, respectively. The increase in cell voltage between the SOA and improved cell resulted in an increase in cell efficiency of 8 percent when the effects of crossover are included.
This work was done by Thomas Valdez and Sekharipuram Narayanan of Caltech for NASA’s Jet Propulsion Laboratory. For further information, access the Technical Support Package (TSP) free on-line at www.techbriefs.com/tsp under the Electronics/ Computers category.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to:
Innovative Technology Assets Management
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109-8099
(818) 354-2240
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Refer to NPO-30829, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Improved Cathode Structure for a Direct Methanol Fuel Cell
(reference NPO-30829) is currently available for download from the TSP library.
Don't have an account?
Overview
The document discusses an innovative cathode structure for Direct Methanol Fuel Cells (DMFC) developed by NASA's Jet Propulsion Laboratory. This new design aims to enhance the performance of DMFCs, particularly at low air flow rates, which is crucial for achieving thermal and water balance while minimizing system size and mass.
The primary challenge addressed is the limited performance of existing membrane-electrode assemblies (MEAs) at low air flow rates, which affects overall efficiency, cell voltage, and power density. The proposed solution involves a novel MEA construction that includes a roughened Nafion membrane surface, a catalyst layer free of hydrophobic particles, and a gas diffusion layer coated with a catalyst containing hydrophobic particles. These components are bonded together under heat and pressure, resulting in a more effective assembly.
The technical disclosure outlines the preparation process for the new MEA, which includes several steps: roughening the Nafion membrane using abrasive paper, applying a catalyst ink mixture, allowing it to dry, and bonding it to a Toray carbon paper backing that has been coated with a catalyst ink. This method differs from traditional techniques by altering the distribution of hydrophobic particles and the pre-treatment of the membrane.
Performance testing of the new MEA shows significant improvements compared to state-of-the-art assemblies. Specifically, the current density at a cell voltage of 0.49 V increased from 75 mA/cm² to 100 mA/cm², resulting in a 50% boost in power density and an enhancement in efficiency from 32% to 39%. This advancement indicates the potential for DMFCs to exceed the energy content of current primary and secondary batteries by 5-10 times, making them a promising alternative for power systems.
The document emphasizes the broader implications of this technology, suggesting that the advancements in DMFCs could lead to more practical and efficient fuel cell systems for various applications beyond aerospace. The research is part of NASA's Commercial Technology Program, aimed at making aerospace-related developments available for wider technological, scientific, and commercial use.

