Nano-engineered catalysts, and a method of fabricating them, have been developed in a continuing effort to improve the performances of direct methanol fuel cells as candidate power sources to supplant primary and secondary batteries in a variety of portable electronic products. In order to realize the potential for high energy densities (as much as 1.5 W•h/g) of direct methanol fuel cells, it will be necessary to optimize the chemical compositions and geometric configurations of catalyst layers and electrode structures. High performance can be achieved when catalyst particles and electrode structures have the necessary small feature sizes (typically of the order of nanometers), large surface areas, optimal metal compositions, high porosity, and hydrophobicity.
The present method involves electrodeposition of one or more catalytic metal(s) or a catalytic-metal/polytetra-fluoroethylene nanocomposite on an alumina nanotemplate. The alumina nanotemplate is then dissolved, leaving the desired metal or metal/polytetrafluoro-ethylene-composite catalyst layer. Unlike some prior methods of making fine metal catalysts, this method does not involve processing at elevated temperature; all processing can be done at room temperature. In addition, this method involves fewer steps and is more amenable to scaling up for mass production.
Alumina nanotemplates are porous alumina membranes that have been fabricated, variously, by anodizing either pure aluminum or aluminum that has been deposited on silicon by electron-beam evaporation. The diameters of the pores (7 to 300 nm), areal densities of pores (as much as 7 × 1010 cm–2), and lengths of pores (up to about 100 nm) can be tailored by selection of fabrication conditions.
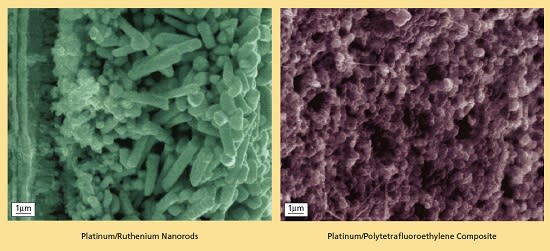
In a given case, the catalytic metal, catalytic metal alloy, or catalytic-metal/ polytetrafluoroethylene composite is electrodeposited in the pores of the alumina nanotemplate. The dimensions of the pores, together with the electrodeposition conditions, determine the sizes and surface areas of the catalytic particles. Hence, the small features and large surface areas of the porosity translate to the desired small particle size and large surface area of the catalyst (see figure).
When polytetrafluoroethylene is included, it is for the purpose of imparting hydrophobicity in order to prevent water from impeding the desired diffusion of gases through the catalyst layer. To incorporate polytetrafluoroethylene into a catalytic-metal/polytetrafluoroethylene nanocomposite, one suspends polytetrafluoroethylene nanoparticles in the electrodeposition solution. The polytetrafluoroethylene content can be varied to obtain the desired degree of hydrophobicity and permeability by gas.
This work was done by Nosang Myung, Sekharipuram Narayanan, and Dean Wiberg of Caltech for NASA’s Jet Propulsion Laboratory.
In accordance with Public Law 96-517, the contractor has elected to retain title to this invention. Inquiries concerning rights for its commercial use should be addressed to:
Innovative Technology Assets Management
JPL
Mail Stop 202-233
4800 Oak Grove Drive
Pasadena, CA 91109-8099
(818) 354-2240
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Refer to NPO-30840, volume and number of this NASA Tech Briefs issue, and the page number.
This Brief includes a Technical Support Package (TSP).

Nano-Engineered Catalysts for Direct Methanol Fuel Cells
(reference NPO-30840) is currently available for download from the TSP library.
Don't have an account?
Overview
The document titled "Nano-Engineered Catalysts for Direct Methanol Fuel Cells" (NPO-30840) from NASA's Jet Propulsion Laboratory outlines a novel fabrication process for catalysts used in direct methanol fuel cells (DMFCs). It addresses the growing demand for high-energy density power sources for portable electronic devices, such as microsensors, cell phones, and laptops, which traditional batteries struggle to meet.
Direct methanol fuel cells are highlighted for their potential to deliver energy densities exceeding 1500 Wh/kg, significantly higher than state-of-the-art primary and secondary batteries, which are limited to 400 Wh/kg and 200 Wh/kg, respectively. The performance of DMFCs is critically dependent on the design of catalyst layers and electrode structures, which influence power density, efficiency, and overall performance.
The document details a new electro-deposition technique that allows for the precise engineering of catalyst nanoparticles and electrode structures. This method utilizes alumina nano-templates to control particle geometry, enabling the production of platinum-ruthenium (Pt-Ru) nano-rods for the anode and platinum-Teflon (Pt-Teflon) nanocomposites for the cathode. The electro-deposition process is advantageous due to its room temperature operation, lower energy requirements, faster deposition rates, and the ability to scale up easily while maintaining uniform deposition at the nanometer scale.
The electroplating process described involves the use of porous alumina templates, which can be fabricated with tunable pore diameters and high pore densities. This allows for the creation of catalysts with ultra-high surface areas, essential for enhancing the performance of fuel cells. The incorporation of Teflon nanoparticles during the electrodeposition of platinum results in nanocomposites that exhibit desirable hydrophobic properties, crucial for gas diffusion electrodes in fuel cells.
Overall, the document presents a significant advancement in the field of fuel cell technology, offering a streamlined and efficient method for producing high-performance catalysts. This innovation not only simplifies the fabrication process but also holds promise for the development of more efficient and powerful energy sources for a variety of applications, paving the way for future technological advancements in portable power solutions.

